two moles of acetyl chloride was mixed with two moles of dimethylamine. after the reaction is complete, what species can be found in the mixture? draw only the organic structures (i.e., omit inorganic ions). show charges and draw any hydrogens on the oxygen or hydrogen atoms where appropriate. 2 moles of acetyl chloride and 2 moles of dimethyl amine react. acetyl chloride is a carbonyl bonded to methyl and chloride. dimethyl amine is a nitrogen bonded to two methyl groups and a hydrogen.
Answers
The reaction between acetyl chloride and dimethylamine yields N, N-dimethylacetamide, an organic compound with a methyl-substituted carbonyl group.
The reaction between two moles of acetyl chloride and two moles of dimethylamine would result in the formation of two moles of the organic compound N, N-dimethylacetamide. Here's the organic structure of N, N-dimethylacetamide:
H
|
H - N - C - O - CH3
| |
CH3 Cl
In this structure, the nitrogen is bonded to two methyl groups and one hydrogen, while the carbonyl carbon is bonded to an oxygen atom and a methyl group.
The chloride atom from acetyl chloride is replaced by the dimethylamine group during the reaction, resulting in the formation of N, N-dimethylacetamide.
Learn more about N,N-dimethylacetamide here:
https://brainly.com/question/15225074
#SPJ4
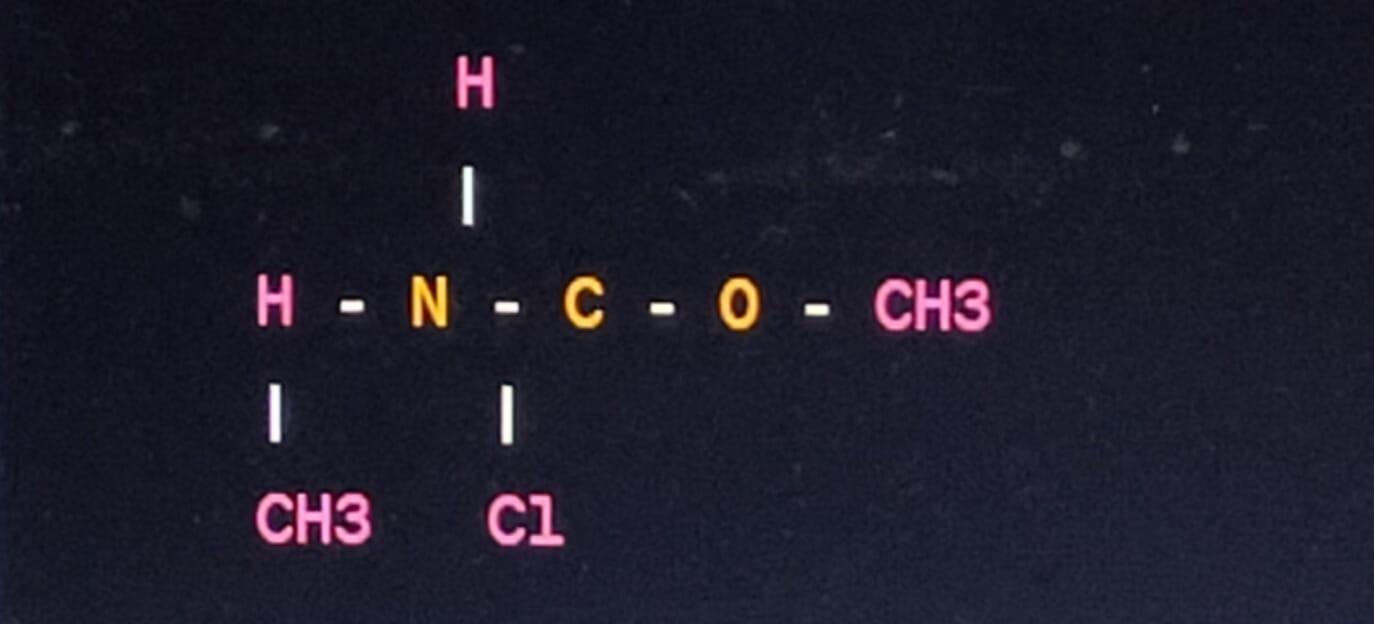
Related Questions
PLEASE HELP !
How does the chemical structure of ionic liquids make them more flexible for scientists to use and what will most likely be the first industrial application of them?
Answers
Explanation:
Ionic liquids offer numerous advantages over conventional organic solvents for carrying out organic reactions, Malhotra notes. "In many cases, product recovery is easier, catalysts can be recycled, and the ionic liquids can be reused," he says. "In addition, their thermodynamic and kinetic behavior is different.
A substance is a solid at room temperature, melts easily, conducts electricity weakly, and dissolves well in water and alcohol. What type of bonds does it have?
Answers
Answer:
Iconic bond
Explanation:
A form of chemical bond that included electrostatic attraction in the oppositely charged ions. It is one of the main types of bonding along with the covalent and metallic bonding. The substance is crystalline in nature since it melts easily and is solid at room temperature.Which of the following proposed reactions are allowed by the law of conservation of electric charge? Select two answers
Answers
The law of conservation of electric charge states that the total electric charge in an isolated system remains constant over time. In other words, the total charge before a reaction should be equal to the total charge after the reaction.
To identify which of the proposed reactions are allowed, we need to examine if the total charge remains constant.
Let's consider two reactions (A and B) as examples:
Reaction A:
Before: 1 positive charge (+1) + 1 neutral charge (0) → After: 2 positive charges (+2) + 1 neutral charge (0)
Total charge before: +1
Total charge after: +2
Reaction B:
Before: 2 positive charges (+2) + 1 negative charge (-1) → After: 1 positive charge (+1) + 1 neutral charge (0) + 1 negative charge (-1)
Total charge before: +1
Total charge after: 0
In reaction A, the total charge before the reaction is not equal to the total charge after the reaction, which violates the law of conservation of electric charge. Therefore, reaction A is not allowed.
In reaction B, the total charge before the reaction is equal to the total charge after the reaction, which obeys the law of conservation of electric charge. Thus, reaction B is allowed.
By applying this principle, you can identify the two proposed reactions that are allowed by the law of conservation of electric charge. Just ensure that the total electric charge remains constant before and after each reaction.
Learn more about law of conservation of electric charge here:
brainly.com/question/9063462
#SPJ11
Radio waves have a lower frequency and energy than X-Rays, so the wavelength of radio waves is than X-Rays.
O shorter
O Exactly half the length of X-Rays
O equal
O longer
Answers
Radio waves have a lower frequency and energy than X-Rays, so the wavelength of radio waves is longer than X-Rays. longer. Option d is correct.
The best approach to compare them is to say that "A gamma ray has more energy than a radio wave because it has a shorter wavelength and a higher frequency."
The most energetic and high frequency particles are gamma rays. On the other side, radio waves are the EM radiation types with the lowest energies, longest wavelengths, and lowest frequencies.
All electromagnetic radiation travels in a vacuum at the speed of light (c), which is the same for all electromagnetic radiation types, including microwaves, visible light, and gamma rays.
Gamma rays contain the maximum energy because they are the strongest members of the electromagnetic spectrum. As a result, they also have a very low frequency and are better able to penetrate solids since they are less likely to interact with matter.
Learn more about gamma ray here
https://brainly.com/question/30689261
#SPJ11
What does the Law of conservation of matter say
Answers
Answer: during a chemical reaction, matter cannot be created or destroyed
Explanation:
how many carbon atoms are in 10.0mg of aspirin C9H8O4 molar mass
180 g mol-1
Answers
There are approximately 0.0004995 carbon atoms in 10.0 mg of aspirin.
The molar mass of aspirin (C9H8O4) is 180 g/mol. Calculate the number of carbon atoms in 10.0 mg of aspirin. The molar mass of C9H8O4 = 9 x atomic mass of C + 8 x atomic mass of H + 4 x atomic mass of O= 9 x 12.011 + 8 x 1.008 + 4 x 15.999= 180.16 g/mol.
Hence, 1 mole of aspirin weighs 180.16 g and contains 9 moles of carbon atoms (1 mole of C9H8O4 contains 9 carbon atoms). Number of moles of aspirin in 10.0 mg = 10.0 mg/180.16 g/mol= 0.0000555 mol. Number of carbon atoms in 10.0 mg of aspirin= 9 x 0.0000555= 0.0004995.
Therefore, there are approximately 0.0004995 carbon atoms in 10.0 mg of aspirin.
Learn more about the "carbon atoms" :
https://brainly.com/question/17154602
#SPJ11
A 3. 8
g sample of sodium hydrogen carbonate is added to a solution of acetic acid weighing 10. 5
g. The two substances react, releasing carbon dioxide gas to the atmosphere. After the reaction, the contents of the reaction vessel weigh 11. 7
g. What is the mass of carbon dioxide released during the reaction?
Answers
The mass of carbon dioxide released during the reaction is 2.6 grams.
To determine the mass of carbon dioxide released during the reaction between sodium hydrogen carbonate (NaHCO3) and acetic acid (CH3COOH), we need to calculate the difference in mass before and after the reaction.
Before the reaction:
Mass of NaHCO3 = 3.8 g
Mass of acetic acid = 10.5 g
Total mass before the reaction = Mass of NaHCO3 + Mass of acetic acid = 3.8 g + 10.5 g = 14.3 g
After the reaction:
Mass of the contents of the reaction vessel = 11.7 g
To find the mass of carbon dioxide released, we calculate the difference in mass:
Mass of carbon dioxide released = Total mass before the reaction - Mass of the contents of the reaction vessel
= 14.3 g - 11.7 g
= 2.6 g
Therefore, the mass of carbon dioxide released during the reaction is 2.6 grams.
learn more about carbon dioxide here
https://brainly.com/question/3049557
#SPJ11
The general formula for an aldehyde is?
Answers
The general formula for an aldehyde is CnH2n+1CHO or CnH2nO.
What is aldehyde?An aldehyde is an organic chemical that contains a functional group with the formula RCH=O. The functional group (without the "R" side chain) is an aldehyde, however it can also be categorized as a formyl group. Aldehydes are widely used and serve vital roles in industry and biology.
Aldehydes have a carbon center that is linked to oxygen by a double bond, hydrogen by a single bond, and a third substituent, which is carbon or, in the case of formaldehyde, hydrogen by a single bond. The middle carbon is frequently described as sp2-hybridized. The aldehyde group is polar in several ways. The length of the C=O bond is around 120-122 picometers.
To learn more about aldehydes visit:
https://brainly.com/question/10287085
#SPJ4
What characterizes a radioactive atom?

Answers
Answer:
It's C
Protons repel the neutrons.
Explanation:
An atom is unstable (radioactive) if these forces are unbalanced; if the nucleus has an excess of internal energy. The instability of an atom's nucleus may result from an excess of either neutrons or protons.
Answer:
B. It's nucleus is unstable
Explanation:
A P E X
Which is a diatomic molecule?
A) Ar
B) CO
C) CO2
D) NaCl
Answers
compare the fuel source and by-products of anaerobic (lactic acid system) and the aerobic energy system.
Answers
The anaerobic system uses glucose as its fuel source and generates lactic acid as a by-product. Meanwhile, the aerobic system uses carbohydrates, fats, and energy and generates energy.
What are the differences between anaerobic and aerobic energy system?Comparing the fuel source and by-products of anaerobic (lactic acid system) and the aerobic energy system, the anaerobic system primarily uses glucose as its fuel source. During high-intensity exercise, the anaerobic system produces energy quickly, but also generates lactic acid as a by-product. This lactic acid can cause muscle fatigue and soreness.
On the other hand, the aerobic energy system uses a combination of carbohydrates, fats, and proteins as its fuel source. It provides energy through a slower, more sustained process, allowing the body to maintain exercise for longer periods. The by-products of the aerobic system are carbon dioxide and water, which are more easily expelled from the body without causing discomfort or fatigue.
In summary, the anaerobic system relies on glucose for rapid energy production but produces lactic acid, while the aerobic system utilizes a mix of carbohydrates, fats, and proteins for sustained energy and produces less harmful by-products.
Learn more about aerobic and anaerobic system here https://brainly.com/question/2655155
#SPJ11
breaking larger molecules into smaller molecules and carbon
Answers
Answer: catabolism
Explanation:
Catabolism refers to the set of metabolic pathways which is necessary for the breaking down of molecules into smaller units. This is then oxidized for the release of energy or can be used to perform other anabolic reactions.
Catabolism is regarded as the opposite direction of anabolism which is simply the building-up of molecules. It should be noted that anabolism and catabolism work together in every living organisms and perform functions such as the production of energy and the repair of cells.
what might be a formula for calculating distance
Answers
Answer:
Distance = speed * Time
hope it helps
Answer:
Distance formula (x2 - a1) ° + (y2 - Yı)?
d - d = distance
(x1, Y1) coordinates of the first point
(x2, Y2) = coordinates of the second point
Which factors most directly affect whether a chemical reaction occurs spontaneously?
Answers
The factors that most directly affect whether a chemical reaction occurs spontaneously are:
1. Temperature: Increasing the temperature generally speeds up chemical reactions. This is because higher temperatures provide more energy to the reactant molecules, increasing their kinetic energy and the likelihood of successful collisions. For example, when heating hydrogen and oxygen gases, they react more rapidly to form water at higher temperatures.
2. Concentration or Pressure: Higher concentrations or pressures of reactant molecules increase the frequency of collisions between them. This leads to a higher chance of successful collisions and thus increases the reaction rate. For instance, increasing the concentration of hydrogen gas in a reaction with nitrogen gas will lead to a faster reaction and the formation of ammonia.
3. Catalysts: Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. They work by providing an alternative reaction pathway with lower activation energy. This allows more reactant molecules to have enough energy to overcome the activation barrier and proceed to the products. For example, enzymes are biological catalysts that speed up chemical reactions in our bodies.
4. Nature of Reactants: The chemical composition and properties of the reactants can also affect whether a chemical reaction occurs spontaneously. Some reactions are more likely to occur because of the inherent instability or reactivity of the reactant molecules. For instance, the reaction between sodium and water is highly exothermic and occurs spontaneously due to the high reactivity of sodium metal.
It's important to note that while these factors influence the rate of a reaction, they don't guarantee that a reaction will occur. The concept of spontaneity in chemical reactions is related to thermodynamics, specifically the change in free energy (∆G) during a reaction. A reaction is spontaneous if ∆G is negative, indicating that the reaction will proceed without any external influence.
Overall, these factors collectively determine whether a chemical reaction occurs spontaneously or not, by affecting the collision frequency, energy, and stability of the reactant molecules.
To know more about chemical reaction :
https://brainly.com/question/34137415
#SPJ11
The factors that most directly affect whether a chemical reaction occurs spontaneously are Temperature, Concentration, Pressure, and Catalysts.
The spontaneity of a reaction is primarily determined by the change in Gibbs free energy (∆G) associated with the reaction. If ∆G is negative, the reaction is spontaneous, while a positive ∆G indicates a non-spontaneous reaction.
The factors that most directly affect whether a chemical reaction occurs spontaneously are the following:
1. Temperature: Increasing the temperature usually increases the rate of a chemical reaction. This is because at higher temperatures, the reactant particles have more kinetic energy, leading to more frequent and energetic collisions. As a result, the reactant molecules are more likely to overcome the activation energy barrier and react.
2. Concentration: Higher concentrations of reactants typically increase the rate of a chemical reaction. This is because when the concentration of reactant particles is higher, there are more collisions between them, leading to a higher probability of successful collisions and therefore an increased reaction rate.
3. Pressure (for gases): For reactions involving gases, increasing the pressure can also increase the reaction rate. This is because higher pressure leads to a higher concentration of gas particles, which in turn increases the frequency of collisions between them.
4. Catalysts: Catalysts are substances that can increase the rate of a chemical reaction without being consumed in the process. They achieve this by providing an alternative reaction pathway with a lower activation energy. By lowering the activation energy, catalysts make it easier for reactant particles to overcome the energy barrier and react, thereby speeding up the reaction.
Know more about chemical reaction:
https://brainly.com/question/22817140
#SPJ11
everything in your body is some type of cell with a
Answers
Provide the systematic name for each of the following isomeric acid chlorides with the chemical formula C6H9ClO.
(Be sure to indicate double bond stereochemistry using (E) and (Z) notation. Indicate stereochemistry in rings with the terms cis or trans. Do NOT use (R) or (S) designations. It is not necessary to use italics in writing compound names. Write compound names in lower case. Use upper case for the double bond stereochemistry terms.)
Answers
The systematic names for the isomeric acid chlorides with the chemical formula C6H9ClO are as follows:
cis-3-chlorocyclopent-1-ene-1-carbonyl chloride
trans-3-chlorocyclopent-1-ene-1-carbonyl chloride
2-chloro-3-methylbut-2-ene-1-carbonyl chloride
The first compound is a cis isomer with a chlorine atom and a carbonyl group on the same side of the cyclopentene ring. The prefix "cis-" is used to indicate this stereochemistry.
The second compound is a trans isomer with a chlorine atom and a carbonyl group on opposite sides of the cyclopentene ring. The prefix "trans-" is used to indicate this stereochemistry.
The third compound is a 2-chloro-3-methylbut-2-ene-1-carbonyl chloride. The chlorine atom is attached to the second carbon atom of the butene chain, and the carbonyl group is attached to the first carbon atom.
The prefix "2-chloro" indicates the position of the chlorine atom, and "3-methyl" indicates the presence of a methyl group on the third carbon atom. The term "but-2-ene-1-carbonyl" describes the butene chain with a carbonyl group attached to the first carbon atom.
Overall, these names provide a clear and systematic description of the isomeric acid chlorides based on their molecular structures and stereochemistry.
For more questions like Isomeric acid click the link below:
https://brainly.com/question/29579764
#SPJ4
write the basic equilibrium equation for po4 3- besure to include the proper phases for all species within the reaction
Answers
The balanced equation for the dissociation of PO43- is given by;PO43-(aq) H2O(l) ⇌ HPO42-(aq) OH-(aq)The phosphate ion, PO43-, reacts with water to form HPO42- (monohydrogen phosphate) and OH- ions.
The chemical equilibrium between these species is represented by the equation above. In the dissociation reaction of PO43- the phosphate ion reacts with water (H2O) to form monohydrogen phosphate ion (HPO42-) and hydroxide ion (OH-). The reaction can be described as;PO43-(aq) H2O(l) ⇌ HPO42-(aq) OH-(aq)where the reactants are on the left and products on the right. In the equation, the state of matter of each reactant or product is written in parenthesis after its chemical formula. In this reaction, the reactant PO43- is an aqueous solution, while H2O is a liquid.
learn more about aqueous solution
https://brainly.com/question/19587902
#SPJ11
You have the same two resistors on a 10 volt series circuit . Will the voltage going into the second resistor be more, less,or the same as that going into the first resistor?
Answers
Answer:
same voltage
Explanation:
Both the resistors will have the same voltage across it and it will be equal to 5 volt.
Now we know if the resistance value is different, then the voltage drop will be different. across them. And the current will be same in both the resistance.
Let suppose, the resistance is 5 ohm on of each one.
Therefore, total current = total voltage / total resistance
= 10 /(5+5) = 10/10 = 1 amp
So now the voltage across the 5 Ohm resistance will be = current × resistance = 1× 5 = 5 volt
Describe the difference
between the smooth
Endoplasmic Reticulum
(ER) and rough
Endoplasmic Reticulum
(ER)
Answers
Answer:
Smooth endoplasmic reticulum (SER) is a type of endoplasmic reticulum consisting of tubular vesicles that lack ribosomes on the outer surface and is involved in the synthesis and storage of lipids. Rough ER has ribosomes on the outer surface. Smooth ER does have riboszomes on the outer surface.
Explanation:
5. (10 Points) Chlorine gas is in a container that has a volume of 25.0 mL at
40°C. What volume will it occupy at 80°C?
PLS HELP
Answers
Answer:120.0mL
Explanation:
Someone plsss help me I will make you as brain plsss
Chemical like polybrominated diphenyl ethers (PBDE) are present in the water in the Great Lakes. These chemicals are toxic when absorbed by the body. Which human health effect can result from the PBDEs in the Great Lakes?

Answers
Answer:
I,lll
Explanation:
Because the human can result from PBDEs in great lakes by water? I think?
Answer:
Its is D. 1,2 and 3
Explanation:
i took the exam already
2. Is the following sentence true or false? Most earthquakes generate
tsunamis.
Answers
I need help w this pls

Answers
Answer:
70..... I think
Explanation:
Cuz if the bus travels 35 km every 30 mins this means that it travels 70 km per hour
why there is only one sodium ion for every chlorine ion, while there are two potassium ions for every oxygen ion?
Answers
There is only one sodium ion for every chlorine ion due to its valency.
The number of electrons that an atom needs to lose or gain in order to achieve a stable electron configuration i.e the octet of an element is known as the valency of an element, which serves as a gauge of that element's potential for combining.
The valency of Sodium (Na) is 1 and that of chlorine (Cl) is also one. therefore they combine to form a stable compound. The same rule follows in the compound formed by Potassium (K) and Oxygen(O) also. The valency of oxygen is 2 which means that it should be surrounded by 2 potassium ions to provide electrons to fulfill both the elements' octets.
To know more about valency, click on https://brainly.com/question/371590
whats white sticky and best not to swallow?
Answers
Answer:
Bro its expired milk duh
Explanation:
PLEASE HELP ASAP!!!!
How is heat related to energy?
A)Heat is a form of energy.
B)Heat and energy are not related.
C)Heat is temperature and energy is fuel.
D)Heat can be created, but energy cannot.
Answers
Heat is related to energy as it is referred to a form of energy which is denoted as option A.
What is Energy?This is also known as thermal energy and it is referred to as the ability to do work and there are different types which include the following below:
Mechanical energyElectrical energyHeat energy.It is also referred to as the form of the energy which is transferred from one body or surface to another as a result of the differences in temperature so as to reach an equilibrium.
Heat energy has various functions such as heating water and electricity generation. It is also lost when energy is transferred from one trophic level to another which is the reason why the energy efficiency is not 100 percent which is therefore the reason why option A was chosen.
Read more about Heat energy here https://brainly.com/question/19666326
#SPJ1
Use the atomic model below to answer the following questions: 1 pt for each blank
How many protons? How many neutrons? What is the name of this atom?
its a k12 test question TwT
Answers
Answer:
Explanation:
ALRIGHT EPIC GAMER IM GONNA INTRODUCE YOU TO THE AWESOME WORLD OF THE PERIODIC TABLE
look at this
6
C
Carbon
12.011
ok so this is what one block on the periodic table looks like
the protons and electrons are going to be the smaller number
so the protons and electrons for carbon are 6
but your question does not ask for electrons so its fine.
How many protons? LOOK AT THE SMALLER NUMBER IN ELEMENT BLOCK ON PERIODIC TABLE.
How many neutrons? Take the bigger number and subtract the smaller number from it. so it would be for carbon: 12.011 - 6 = 12 neutrons.
to find the name of the atom, just count the number of electrons and then look for the element with the same number to find the name
Explanation of the concepts of mole ratio in stoichiometry to calculate theoretical yield. Support the concept with:an explanation of the importance of considering mole ratios in two different commercial or industrial chemical processes.provide one example including a relevant equation and calculations to support the explanation. Briefly discuss the effects of limiting and excess reagents in this reaction.
Answers
Mole ratio is the proportional amount of moles of two or more compounds in a chemical reaction, and this concept is widely used in Stoichiometry, since in this type of matter we have to be comparing initial amount of reactant and final amount of product, usually, we have to convert grams of mass into moles of the compound, and when we have to compare the number of moles of different compounds, we use mole ratio, as we can see in the example below:
A + 2 B -> X
The mole ratio between A and B is 1:2, therefore if we have 3 moles of A in the reaction, we would have 6 moles of B, and with that concept in mind, we can find the mass asked in any question.
In this process, we can have the theoretical yield of a reaction, which is how much of the product is produced from the initial amount of reactant
We have two oversimplified reactions that take in account mole ratio:
1. Formation of Ammonia:
N2 + 3 H2 -> 2 NH3, here we have the following mole ratios:
1 N2 = 3 H2
1 N2 = 2 NH3
3 H2 = 2 NH3
2. Burning of Octane, which is fuel:
2 C8H18 + 25 O2 -> 16 CO2 + 18 H2O
The mole ratios are:
2 C8H18 = 25 O2
2 C8H18 = 16 CO2
2 C8H18 = 18 H2O
25 O2 = 16 CO2
25 O2 = 18 H2O
16 CO2 = 18 H2O
Limiting and excess reactants are, as the name already implies, the reactant the will limit the amount of the other reactant undergoing the reaction, and this is found through mole ratio. The excess reactant is the reactant that will not totally react, but it will have some of it left without undergoing the reaction
(c) The figure below shows two alkane molecules.
Methane
HIC-H
HICIH
Methane
Hexane
Hexane
HHHHHH
LIITIT
H-C-C-C-C-C-C-H
IITTIT
H. H HHHH.
The table below shows the melting points and boiling points of methane and
hexane.
Melting point in Boiling point in
°C
°C
-183
-95
-162
69
Compare the structure and properties of methane and hexane.
(1)
Answers
Methane is a gas which is colourless and and odorless. It is a hydrocarbon with formula CH₄. Where as Hexane is an alkane containing 6 carbon atoms. Its molecular formula is C₆H₁₄. For the structure refer the figure given below.
Coming to the properties there are both physical and chemical properties.
Hexane is a colourless volatile liquid and undergoes combustion reaction, being a higher hydrocarbon it undergo thermal cracking.
It is commonly used as solvents, biodiesel production etc. Boiling point is 69⁰C and melting point is 95⁰C.
Methane is a gas which is colourless and odorless. It is lighter in air, slightly soluble in water, it is flammable. boiling point is -162⁰C and melting point is -183⁰C.It is used as fuel, it is used in explosives.
Learn more about alkanes here https://brainly.com/question/1621859
#SPJ9

what is the heat capacity of a calorimeter in j/oc if 846.4 j of heat raises its temperature by 8.5 oc?
Answers
The heat capacity of the calorimeter is 99.52 J/°C.
The heat capacity of a calorimeter in j/°C if 846.4 J of heat raises its temperature by 8.5 °C is given by:
Heat capacity = Q / ΔT
where Q is the heat energy supplied to the calorimeter and ΔT is the corresponding increase in temperature.
Thus, substituting the given values:
Heat capacity = 846.4 J / 8.5 °C = 99.52 J/°C
Therefore, the heat capacity of the calorimeter is 99.52 J/°C.
To know more about heat capacity, refer here:
https://brainly.com/question/28921175#
#SPJ11