The volume of a sample of hydrogen gas at 0. 997 atm is 5. 00 L. What will be the new volume if the pressure is decreased to 0. 977 atm?
Answers
The new volume of the hydrogen gas is 5.12 L when the pressure is decreased to 0.977 atm.
The relationship between pressure and volume is described by Boyle's Law, which states that when the pressure of a gas decreases, its volume increases proportionally, and vice versa. In other words, the pressure and volume of a gas are inversely proportional, assuming temperature and amount of gas remain constant.
In this case, the initial pressure of the hydrogen gas is 0.997 atm, and its initial volume is 5.00 L. If the pressure is decreased to 0.977 atm, we can use Boyle's Law to calculate the new volume:
P1V1 = P2V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the new pressure and volume.
Substituting the given values, we get:
(0.997 atm)(5.00 L) = (0.977 atm)(V2)
Solving for V2, we get:
V2 = (0.997 atm)(5.00 L) / (0.977 atm)
V2 = 5.12 L
Therefore, the new volume of the hydrogen gas is 5.12 L when the pressure is decreased to 0.977 atm.
To know more about hydrogen gas, visit:
https://brainly.com/question/12494649#
#SPJ11
Related Questions
Using the following equation
2C2H6 +7O2 -->4CO2 +6H2O
How many grams of C2H6 are formed from 9.5 moles of CO2 gas?
Answers
30.06904 grams of C2H6 are formed from 9.5 moles of CO2 gas.
How to find number of moles?
In the International System of Units, the mole is the unit of substance amount. A mole of a substance is defined as a mass of material that contains exactly 12,000 g of 12C's exact number of atoms as fundamental units. One mole has 600 sextillion molecules. While employing the mole, complicated calculations are more easily understandable. To get the number of moles, divide the compound's known mass by its molar mass. Consider a scenario where your sample of Na2SO4 weighs 20 g. 20 grammes divided by 142 grammes per mole yields 0.141 moles.
To know more about moles, refer: -
https://brainly.com/question/14276478
SPJ1
Which of the following choices is an
example of gathering evidence
Answers
Answer: Taking measurements
Explanation:
Apex
Balance the chemical equation. Values may be used more than once.
Ga + H2SO4 ⇒ Gaz(SO4)3 + H2
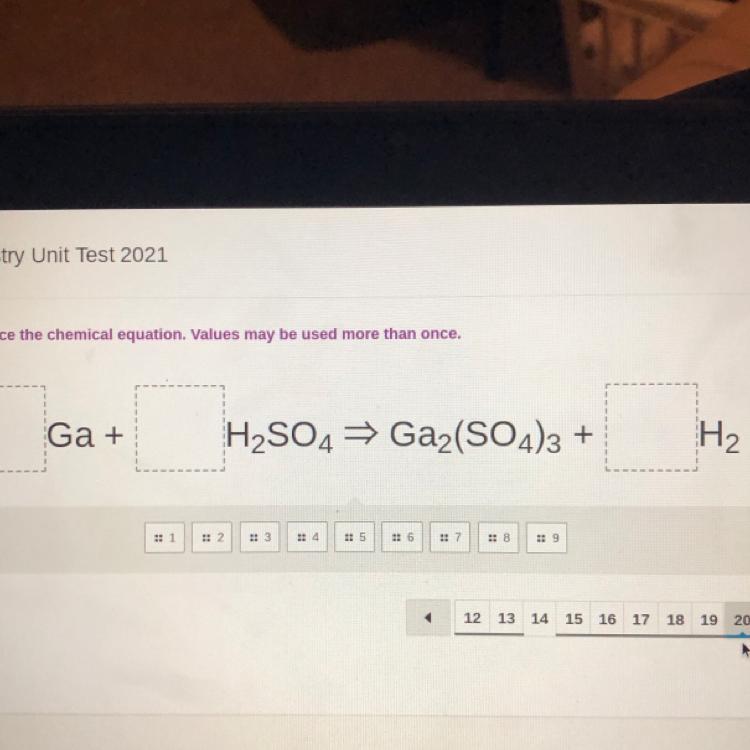
Answers
\(\\ \sf\longmapsto Ga+H_2SO_4\longrightarrow Ga_2(SO_4)_3+H_2\)
Balanced equation:-
\(\\ \sf\longmapsto 2Ga+3H_2SO_4\longrightarrow Ga_2(SO_4)_3+3H_2\)
On reactant side
Ga=2H=6SO_4=3On products side
Ga=2H=6SO_4=3Hence balanced
Chemistry help needed. Correct answer only pls! Need it done by Sunday

Answers
1. The molarity of the solution is 0.674 M.
2. 47.88 g of CuSO₄ in a small volume of water to make a concentrated solution.
3. 217 mL of 6.00M H₂SO₄ using a graduated cylinder or pipette, and transfer it to a 500 mL volumetric flask.
1. The molar mass of KNO₃ is:
K = 39.10 g/mol
N = 14.01 g/mol
O = 16.00 g/mol (x3)
Molar mass of KNO₃ = 101.10 g/mol
To find the number of moles of KNO₃:
mass = 341 g
moles = mass/molar mass = 341/101.10 = 3.37 mol
The volume of the solution is given as 5.0 L, so the molarity of the solution is:
Molarity = moles of solute/volume of solution
Molarity = 3.37 mol/5.0 L = 0.674 M.
2. To prepare 250 mL of 1.2M CuSO₄ solution:
1.2 mol/L x 0.250 L = 0.30 mol CuSO₄
Then, calculate the mass of CuSO₄ needed using its molar mass:
0.30 mol x 159.61 g/mol = 47.88 g CuSO₄
3. To prepare 500 mL of 2.6M H₂SO₄ solution:
2.6 mol/L x 0.500 L = 1.3 mol H₂SO₄
Then, calculate the volume of 6.00M H₂SO₄ needed to contain 1.3 mol of H₂SO₄:
1.3 mol / 6.00 mol/L = 0.217 L = 217 mL
To learn more about the molarity, follow the link:
https://brainly.com/question/31545539
#SPJ1
Find the standard molar enthalpy for the reaction C(s) + ½ O2(g) → CO(g)
Answers
The standard molar enthalpy for the reaction C(s) + ½ O2(g) → CO(g) is -111KJ.
What exactly are molar enthalpy and enthalpy?Molar enthalpy is the amount of energy per mole. In light of this, the primary distinction between enthalpy and molar enthalpy is that the former refers to the total heat content of a thermodynamic system, whereas the latter refers to the total heat per mole of reactant in the system.
C(s) + O₂(g) → CO₂(g) ΔHO = -394 kJ ----(1)
CO₂(g) → CO(g) + 1/2O₂(g) ΔHO = +283 kJ -----(2)
Adding 1 & 2
C(s) + ½ O₂(g) → CO(g)
ΔHO = -394 kJ + 283 kJ
ΔHO = -111KJ.
To know more about molar enthalpy visit:
https://brainly.com/question/3207013
#SPJ1
Complete question is " Find the standard molar enthalpy for the reaction C(s) + ½ 02(g) → CO(g)
Given that
C(s) + O2(g) → CO2(g) AHO = -394 kJ
CO2(g) → CO(g) + ¹/2O2(g) AHO = +283 kJ ".
how does a conjugate acid differ from its conjugate base? select all that apply. multiple select question. the conjugate acid has one more h than its conjugate base. a conjugate base may be positively charged, neutral, or negatively charged. the conjugate acid will be more negatively charged than its conjugate base. the conjugate acid is the substance that reacts with the original base in an acid-base reaction. the conjugate acid of a neutral base will have a charge of 1.
Answers
The conjugate acid has one more h than its conjugate base. A conjugate base may be positively charged, neutral, or negatively charged.
What is conjugate acid and conjugate base?Conjugate acids and bases are ideas included in the Bronsted-Lowry acid-base theory. An acid loses a hydrogen ion as it splits into its ions in water. The conjugate base of the acid is the species that results.
In a chemical process, the conjugate base has the capacity to either acquire or absorb a proton. The proton and hydrogen is given away in the process by the conjugate acid. The conjugate acid has one more h than its conjugate base. A conjugate base may be positively charged, neutral, or negatively charged.
Therefore, the conjugate acid has one more h than its conjugate base. A conjugate base may be positively charged, neutral, or negatively charged.
To learn more about conjugate acid and conjugate base, here:
https://brainly.com/question/12883745
#SPJ1
What is the isotope name of the element that has 9 neutrons?
1)Beryllium-4
2 ) Beryllium-9
3) Fluorine-9
4) Fluorine-19
5)Not enough information to tell
Answers
We do not have enough information to deduce the isotope that would have nine neutrons. Option 5
What is the neutron?The atom is composed of subatomic particles. The subatomic particles that are in the atom are such that we have the electrons that are negatively charged, the protons that are positively charged and the neutrons that are uncharged.
Now, we know that the number of neutrons is obtained as the difference between the mass number and the atomic number of the atom. In this case, we only have the mass numbers and not the atomic numbers thus we do not have sufficient information.
Learn more about neutrons:https://brainly.com/question/28992636
#SPJ1
Describe the Chemiosmotic Hypothesis (aka the Mitchell Hypothesis). Be sure to include the following terms in your description: NADH, FADH2, electrons, protons, inner mitochondrial membrane, and ATP synthase. Explain how an overdose of aspirin impacts the Chemiosmotic Hypothesis and the reduction of O2 to H2O in the electron transport chain.
Answers
The Chemiosmotic Hypothesis, also known as the Mitchell Hypothesis, is a theory proposed by Peter Mitchell in 1961 that explains the mechanism by which ATP (adenosine triphosphate) is synthesized in the mitochondria during cellular respiration. It describes the coupling of electron transport and proton movement across the inner mitochondrial membrane to generate ATP.
During cellular respiration, NADH (nicotinamide adenine dinucleotide) and FADH2 (flavin adenine dinucleotide) are produced as electron carriers in the citric acid cycle (also known as the Krebs cycle) and glycolysis. These electron carriers donate electrons to the electron transport chain (ETC) located on the inner mitochondrial membrane.
As electrons pass through the ETC, they move from protein complex to protein complex, releasing energy in the process. This energy is used to actively pump protons (H+) from the mitochondrial matrix to the intermembrane space, creating an electrochemical gradient across the inner mitochondrial membrane.
The inner mitochondrial membrane is impermeable to protons, so their movement back into the mitochondrial matrix can only occur through a specialized enzyme called ATP synthase. ATP synthase acts as a molecular turbine and allows protons to flow back into the matrix, using their energy to drive the synthesis of ATP from ADP (adenosine diphosphate) and inorganic phosphate (Pi). This process is known as oxidative phosphorylation.
The reduction of oxygen (O2) to water (H2O) is the final step in the ETC. Oxygen acts as the final electron acceptor, receiving electrons and protons to form water. This process is essential for maintaining the flow of electrons through the ETC and the production of ATP.
When an overdose of aspirin occurs, it can impact the Chemiosmotic Hypothesis and the reduction of O2 to H2O in the following ways:
Inhibition of ATP synthase: Aspirin can inhibit the activity of ATP synthase, preventing the flow of protons back into the mitochondrial matrix. This disrupts the chemiosmotic gradient and impairs ATP synthesis, leading to a decrease in ATP production.Disruption of electron transport: Aspirin can also interfere with the transfer of electrons within the ETC. By inhibiting specific enzymes, aspirin can disrupt the normal flow of electrons and disrupt the reduction of O2 to H2O. This can lead to a buildup of partially reduced intermediates, potentially causing cellular damage.Overall, the overdose of aspirin affects the chemiosmotic coupling of electron transport and proton movement, leading to a decrease in ATP synthesis and potential disruption of the reduction of O2 to H2O in the electron transport chain.
To learn more about ATP synthase , visit:
https://brainly.com/question/893601
#SPJ11
Which term refers to a substance that changes color when there is a certain concentration of hydrogen ions in a solution
Answers
Chemical Indicator refers to a substance that changes color when there is a certain concentration of hydrogen ions in a solution.
Chemical indicators are any substances that provide a clear indication—typically a change in color—of the presence or absence of a certain chemical species, like an acid or an alkali, in a solution. One such chemical is methyl yellow, which gives an alkaline solution a yellow color. When introduced to acidic or alkaline solutions, substances are considered indicators when their color changes. There are several indicators that are frequently used in laboratories, including litmus, phenolphthalein, and methyl orange. Litmus paper: It turns red in acidic solutions and blue in basic ones. Methyl orange: This chemical exhibits a red color in an acidic solution and a yellow color in a basic solution.
Learn more about Chemical indicators
brainly.com/question/13748767
#SPJ4
Calculate the settling velocity of a particle with 10 µm diameter and a specific gravity of 1.05 in 15 ˚C water (1.140 x 10^ -3 N-s / m^s and the density of water is 999.1 kg / m^3).
Answers
For a 10 µm diameter particle with a specific gravity of 1.05 in 15 ˚C water, the settling velocity can be determined using Stoke's law.
The settling velocity of a particle can be calculated using Stoke's Law, which is given by the equation:
\(V = (2/9) * (g * (ρp - ρf) * d^2) / η\)
Where:
V is the settling velocity,
g is the acceleration due to gravity (approximately 9.81\(m/s^2\)),
ρp is the density of the particle,
ρf is the density of the fluid,
d is the diameter of the particle, and
η is the dynamic viscosity of the fluid.
Given that the diameter of the particle is 10 µm (or \(10 x 10^-6 m\)) and the specific gravity is 1.05, we can calculate the density of the particle using the equation:
ρp = ρf * (specific gravity)
Substituting the values, we have ρp = (999.1 \(kg/m^3\)) * 1.05 = 1049.545 \(kg/m^3\).
Using the known values of ρp, ρf (density of water), d, and the dynamic viscosity of water at 15 ˚C (\(1.140 * 10^ -3 N-s/m^2\)), we can substitute these values into the Stoke's Law equation to calculate the settling velocity of the particle.
Learn more about velocity here:
https://brainly.com/question/17005285
#SPJ11
a chemistry graduate student is given of a pyridine solution. pyridine is a weak base with . what mass of should the student dissolve in the solution to turn it into a buffer with ph ? you may assume that the volume of the solution doesn't change when the is dissolved in it. be sure your answer has a unit symbol, and round it to significant digits.
Answers
The mass of C₅H₅NHCl , the student should be dissolve in the solution to turn it into a buffer with ph = 5.64 is equals to the 31.45g.
We have a pyridine solution, which is a weak base solution. For a weak base,
the ionization constant, kb = 1.7 × 10⁻⁹
Buffer pH = 5.64
Volume of solution = 250 mL
Molarity of solution = 0.7 M
Using pH formula, pKb = - log ( 1.7 × 10⁻⁹)
= 8.77
For pH of equilibrium constant of water,
pKw = pKb + pKa
=> 14 = 8.77 + pKa
=> pKa = 5.23
Buffer pH formula = pKa + log( [[C₅H₅N] /[C₅H₅NHCl])
=> 5.64 = 5.23 + log( 0.7 M/[C₅H₅NHCl])
=> log(0.7 M/[C₅H₅NHCl]) = 5.64 - 5.23
=> log(0.7 M/[C₅H₅NHCl]) = 0.41
So, 0.7 M/[C₅H₅NHCl] = 10⁻⁰·⁴¹
=> [C₅H₅NHCl] = 10⁻⁰·⁴¹ × 0.7 M
Moles of [C₅H₅NHCl] = 250 mL× 10⁻⁰·⁴¹ × 0.7 mol [C₅H₅NHCl] / 1000 mL
= (0.7 ×10⁻⁰·⁴¹)/4
= 0.175 ×10⁻⁰·⁴¹ moles = 0.2723 moles.
Molar mass of C₅H₅NHCl = 115.5 g/mol
Mass of [C₅H₅NHCl] = 0.175 ×10⁻⁰·⁴¹ moles × 115.5 g [C₅H₅NHCl]/ 1 mol of [C₅H₅NHCl]
= 0.2723 × 115.5 g = 31.454 g
Hence, required mass is 31.45 g.
For more information about buffer pH, visit :
https://brainly.com/question/2288798
#SPJ4
Complete question:
a chemistry graduate student is given 250 ml of a 0.7 M pyridine solution C5H5N. pyridine is a weak base with Kb = 1.7 × 10-9. what mass of C5H5NHCl should the student dissolve in the solution to turn it into a buffer with ph= 5.64 ? you may assume that the volume of the solution doesn't change when the is dissolved in it. be sure your answer has a unit symbol, and round it to significant digits.
How much heat is required to heat 50.0 grams of water from 0° C to 40.0° C, if the specific heat of water is 4.18 J/(g·°C)?
a. 200 kJ
b 2000 J
c. 8360 J
d. 836 kJ
Answers
Answer:
Explanation:
In order to be able to solve this problem, you will need to know the value of water's specific heat, which is listed as
c
=
4.18
J
g
∘
C
Now, let's assume that you don't know the equation that allows you to plug in your values and find how much heat would be needed to heat that much water by that many degrees Celsius.
Take a look at the specific heat of water. As you know, a substance's specific heat tells you how much heat is needed in order to increase the temperature of
1 g
of that substance by
1
∘
C
.
In water's case, you need to provide
4.18 J
of heat per gram of water to increase its temperature by
1
∘
C
.
What if you wanted to increase the temperature of
1 g
of water by
2
∘
C
? You'd need to provide it with
increase by 1
∘
C
4.18 J
+
increase by 1
∘
C
4.18 J
=
increase by 2
∘
C
2
×
4.18 J
To increase the temperature of
1 g
of water by
n
∘
C
, you'd need to supply it with
increase by 1
∘
C
4.18 J
+
increase by 1
∘
C
4.18 J
+
...
=
increase by n
∘
C
n
×
4.18 J
Now let's say that you wanted to cause a
1
∘
C
increase in a
2-g
sample of water. You'd need to provide it with
for 1 g of water
4.18 J
+
for 1 g of water
4.18 J
=
for 2 g of water
2
×
4.18 J
To cause a
1
∘
C
increase in the temperature of
m
grams of water, you'd need to supply it with
for 1 g of water
4.18 J
+
for 1 g of water
4.18 J
+
,,,
=
for m g of water
m
×
4.18 J
This means that in order to increase the temperature of
m
grams of water by
n
∘
C
, you need to provide it with
heat
=
m
×
n
×
specific heat
This will account for increasing the temperature of the first gram of the sample by
n
∘
C
, of the the second gram by
n
∘
C
, of the third gram by
n
∘
C
, and so on until you reach
m
grams of water.
And there you have it. The equation that describes all this will thus be
q
=
m
⋅
c
⋅
Δ
T
, where
q
- heat absorbed
m
- the mass of the sample
c
- the specific heat of the substance
Δ
T
- the change in temperature, defined as final temperature minus initial temperature
In your case, you will have
q
=
100.0
g
⋅
4.18
J
g
∘
C
⋅
(
50.0
−
25.0
)
∘
C
q
=
10,450 J
Rounded to three sig figs and expressed in kilojoules, the answer will be
please help with the attached picture question 21, 22, 23

Answers
Explanation:
21. The given molecule for cracking is tetradecane.
On cracking it forms one mole of decane (C10H22) and two moles of ethene gas.
The chemical equation is shown below:
\(C_1_4H_3_0->C_1_0H_2_2+2C_2H_4\)
22. The essential condition for the formation of an ester is the reaction of alcohol and acid in presence of concentrated sulfuric acid.
Thus among the given options, the first option is the correct one.
23. Isomers of butanol are shown below:
It is 2-butanol.
The position of -OH group changes to the second carbon.

Individuals in this stage of change may sporadically engage in physical activity but without any form, structure, or consistency.
Select one:
a. Maintenance
b. Precontemplation
c. Preparation
d. Contemplation
Answers
Individuals who sporadically engage in physical activity without form, structure, or consistency are in the " Precontemplation" stage of change.
The correct answer is b.
Individuals in the pre-contemplation stage of the Transtheoretical Model of Behavior Change have no intention of changing their behavior in the near future.
They may be unaware of the need for change or may feel resigned to their current behavior. In terms of physical activity, individuals in this stage may engage in sporadic or irregular activity, but they are not yet considering making exercise a regular part of their lifestyle.
Therefore option b is correct.
Learn more about behavior:
https://brainly.com/question/1741474
#SPJ11
can you go from an aldehyde to a carboxylic acid
Answers
Producing Carboxylic Acids from Primary Alcohols or Aldehydes via Oxidation.The potassium dichromate(VI) solution undergoes a reaction and changes colour from orange to green.
An organic substance with a carboxyl functional group is known as a carboxylic acid. They are extensively distributed in nature and are also produced artificially by people. When carboxylic acids deprotonate, a carboxylate anion with the general formula R-COO- is produced. This anion can be used to create a number of beneficial salts, including soaps. Because it is made up of two functional groups—a carboxyl group and a hydroxyl group attached to a carbonyl group—the carboxyl functional group that distinguishes carboxylic acids is uncommon. It is frequently written as -CO2H or -COOH in condensed form. An organic acid that has a carboxyl group (C(=O)OH) connected to an R-group is referred to as a carboxylic acid in organic chemistry. A carboxylic acid's usual formula is RCOOH or RCO.
Learn more about Carboxylic Acids here:
https://brainly.com/question/29035899
#SPJ4
What is the approximate frequency of green light that has a wavelength of 520 nm?

Answers
Answer:
5.77 x 10¹⁴ Hz
Explanation:
A calcium ion will commonly lose 2 electrons to form an ion. When a calcium (Ca) ion is formed, what is its charge and why?
A calcium ion will form a [ 1+ OR 1-] charge because it will have...
-more neutrons than electrons
-more electrons than protons
-more protons than electrons
Answers
Answer:
A calcium ion will form a 1+ charge because it will have more protons than electrons.
Explanation:
Electrons have negative charges
Protons have positive charges
Calcium likes to lose its extra electron to achieve an octet outer shell and be "happy".
write down the cell equation. please

Answers
Answer:
see explanation
Explanation:
symbiology is (oxidation rxn || reduction rxn)
=> Zn°|Zn⁺² || Cu⁺²|Cu° => Zn°(s) + Cu⁺²(aq) => Zn⁺²(aq) + Cu°(s)
phosphorus-32 has a half-life of 14.3 days. if an initial sample has a mass of 4.00 mg, how many milligrams will remain after 71.5 days?
Answers
Answer:0.125 g
Explanation:
HELP PLEASE CHEMISTRY

Answers
a. There are 0.1596 moles of NaCl in 0.3 L of NaCl stock solution.
b. There are 0.1596 moles of NaCl in 2.1 L of NaCl dilute solution.
c. The concentration of NaCl in the final solution is 0.076 M.
Give a brief account on molarity.Molarity is said to be the number of moles of solute per liter of solution. For example, when salt is dissolved in water, the salt becomes the solute and the water becomes the solution. Since, one mole of sodium chloride weighs 58.44 grams and dissolving 58.44 grams of NaCl in 1 liter of water makes a 1 molar solution, abbreviated as 1M.
c. Let's calculate the concentration of NaCl in the final solution:
M₁V₁ = M₂V₂
M₁ = Initial concentration of NaCl (0.532 M)
V₁ = Initial volume of NaCl (0.3 L)
M₂ = Final concentration of NaCl
V₂ = Initial volume of NaCl (2.0 L)
0.532 × 0.3 = M₂ × 2.1
M₂ = (0.532 × 0.3)/2.1
M₂ = 0.076 M
a. To calculate number of moles in 0.3 L of NaCl stock solution.
Molarity = Mole of solute/Volume of solution
0.532 = Mole of NaCl/0.3
Mole of NaCl = 0.532×0.3
Mole of NaCl = 0.1596 mol
b. To calculate number of moles in 2.1 L of NaCl solution.
0.076 = Mole of NaCl/2.1
Mole of NaCl = 0.076×2.1
Mole of NaCl = 0.1596 mol
To know more about Molarity, visit:
https://brainly.com/question/8732513
#SPJ1
please help :) How can scientists ensure that their data are reliable? A) by making a single observation B) by recording values without units C) by keeping the results private D) by repeating trials during an experiment
Answers
Answer:
D. by repeating trials during an experiment
Explanation:
chile its actually d
Explanation:
which molecule or ion has a trigonal planar geometry around its central atom?
Answers
Answer:
Structure of boron trifluoride, an example of a molecule with trigonal planar geometry.
Explanation:
35.0 grams of nitrogen gas reacts with 60.0 grams of hydrogen gas: N2 + 3H2--> 2NH3
a) Identify the limiting reagent:
b) Calculate the grams of ammonia formed:
c) Calculate the grams of excess reactant formed:
Answers
Explanation:
Moles of N2 = 35.0g / (28g/mol) = 1.25mol
Moles of H2 = 60.0g / (2g/mol) = 30.0mol
Since 1.25mol * 3 < 30.0mol, nitrogen is limiting.
Moles of NH3 = 1.25mol * 2 = 2.50mol.
Mass of NH3 = 2.50mol * (17g/mol) = 42.5g.
30.0mol - 1.25mol * 3 = 26.25mol.
Excess mass of H2
= 26.25mol * (2g/mol) = 52.5g.
1. What i the advantage of making unblock lightly non polar? Provide a full explanation of the chemical principle involved
Answers
The advantage of making a solvent unblocking lightly nonpolar is to increase the solubility of polar and ionic compounds.
The solubility of a solute in a solvent is largely determined by the relative polarity of the solute and solvent. Polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Making a solvent unblock lightly nonpolar increases its ability to dissolve polar and ionic compounds by reducing the difference in polarity between the solvent and solute.
This allows polar and ionic compounds to be dissolved in a nonpolar solvent, which can be useful in various applications, such as chromatography and chemical reactions.
Learn more about polar and ionic compounds:
https://brainly.com/question/24692494
#SPJ4
A 10.0-mL solution of 0.300 M NH3 is titrated with a 0.100 M HCl solution. Calculate the pH after the following additions of the HCl solution: (a) 0.0 mL, (b) 10.0 mL, (c) 30.0 mL, (d) 40.0 mL
Answers
The pH values of the solution after the addition of various amounts of acids are as follows:
(a) When 0.0 mL HCl is added; pH = 11.62
(b) After adding 10.0 mL of 0.100 M HCl; pH = 12.38
(c) After adding 30.0 mL of 0.100 M HCl; pH = 14
(d) After adding 40.0 mL of 0.100 M HCl; pH = 2.70
What are the pH values of the solution after the addition of various amounts of acids?The equation of the reaction between NH₃ and HCl is:
NH₃ + HCl → NH₄Cl
Before the addition of HCl:
The concentration of NH₃ solution = 0.300 M
The initial concentration of OH- can be calculated from the Kb of NH₃ :
Kb = [NH₄⁺][OH⁻] / [NH₃]
1.8 x 10⁻⁵ = x² / 0.300
x = 0.0024 M
The initial concentration of OH⁻ is 0.0024 M, and the initial pH of the solution ill be:
pH = 14 - pOH
pH = 14 - (-log[OH-])
pH = 11.62
(a) When 0.0 mL HCl is added;
pH = 11.62
(b) After adding 10.0 mL of 0.100 M HCl;
moles of HCl added = (0.100 mol/L) x (0.0100 L)
moles of HCl added = 0.00100 mol
The moles of NH₃ initially present = (0.300 mol/L) x (0.0100 L)
moles of NH₃ initially present = 0.00300 mol
the mole ratio of HCl and NH₃ = 1:1
The remaining moles of NH₃ = 0.00300 mol - 0.00100 mol
remaining moles of NH₃ = 0.00200 mol
The volume of the solution is now 10.0 mL + 10.0 mL = 20.0 mL or 0.0200 L.
The new concentration of NH₃ = 0.00200 mol / 0.0200 L
concentration of NH₃ = 0.100 M
The concentration of OH- can be calculated from the Kb of NH₃ using the new concentration of NH4+:
Kb = [NH4+][OH-] / [NH3]
1.8 x 10⁵ = x² / 0.100
x = 0.0042 M
The new concentration of OH- is 0.0042 M, and the new pH will be:
pH = 14 - (-log[OH-])
pH = 12.38
(c) After adding 30.0 mL of 0.100 M HCl;
the volume of the solution is 40.0 mL or 0.0400 L
The moles of HCl added = (0.100 mol/L) x (0.0300 L)
moles of HCl added = 0.00300 mol
moles NH3 reacted = 0.00300 mol - 0.00300 mol
moles NH3 reacted = 0.00000 mol
[NH₄⁺] remaining [OH⁻] = 0
The concentration of OH- is 0 M, so the pH = 14 - (-log[OH-])
pH = 14
(d) After adding 40.0 mL of 0.100 M HCl;
moles HCl = (0.100 mol/L) x (0.0400 L)
moles HCl = 0.00400 mol
The volume of solution = 0.050 L
All NH₃ has reacted and the remaining moles of HCl = (0.00400 mol - 0.00300 mol) / 0.0500 L
the remaining moles of HCl = 0.00200 M
The concentration of H₃O⁺ can be calculated from the concentration of HCl:
0.00200 M HCl produces 0.00200 M H₃O⁺
pH = -log[H₃O⁺]
pH = -log(0.00200)
pH = 2.70
Learn more about pH at: https://brainly.com/question/172153
#SPJ1
Once a peptide has been formed between the amino acid attached to the trna in the p site and the amino acid associated with the trna in the a site, which process on the list occurs next?.
Answers
The complex known as RIBOSOME, which is made up of enzymes and ribosomal RNA, is where translocation peptide has indeed been generated.
What age is the ideal time to begin taking peptides?
Since collagen production normally slows down in your mid-20s to early-30s, I advise incorporating a peptide into your skincare regimen about this time. But regardless of your age, incorporating a peptide into your skincare regimen will make your skin look younger.
Peptide vs. protein: what are they?
In general, small chains of more than two amino acids are referred to as peptides. Proteins, commonly known as polypeptides, are lengthy molecules comprised of many peptide subunits. Enzymes (other proteins) can break down proteins into little peptide fragments.
To know more about peptide visit:
https://brainly.com/question/14902522
#SPJ4
Select the correct structure that
corresponds to the name.
4-bromo-5-chlorocyclohexene
Answers
Answer: a
Explanation:
While visiting his uncle's farm, Derek learned that horses and donkeys are two different species. Based on this
information, what can Derek infer about horses and donkeys?
Horses and donkeys cannot survive in the same
environment.
Horses and donkeys produce fertile offspring.
Horses and donkeys are members of the same
population.
Horses and donkeys are members of different
populations.
Answers
While visiting his uncle's farm, Derek learned that horses and donkeys are two different species. Based on this information, Derek can infer that horses and donkeys are members of different populations.
Since horses and donkeys are different species, they belong to different populations. A population refers to a group of individuals of the same species that live in the same area and can interbreed. While horses and donkeys can mate, their offspring, known as mules, are usually infertile.
This means that mules cannot produce offspring of their own, which indicates that horses and donkeys are not members of the same population. In contrast, if they were members of the same population, they would be able to produce fertile offspring. Therefore, Derek can infer that horses and donkeys are members of different populations.
Learn more about mules here:
https://brainly.com/question/15852289
#SPJ11
All the following would be expected to affect the rate of a chemical reaction EXCEPT a. adding more reactants.b. removing some products. c. increasing the temperature. d. decreasing the temperature. e. adding a catalyst.
Answers
A chemical reaction is a process where reactants are transformed into products.The rate of a chemical reaction can be affected by different factors, such as adding more reactants, removing some products, increasing the temperature, decreasing the temperature, and adding a catalyst.
Adding more reactants would increase the rate of the reaction since there would be more reactants available to react with each other. Removing some products would also increase the rate of the reaction since it would drive the reaction forward by reducing the concentration of products. Increasing the temperature would increase the rate of the reaction since it would increase the kinetic energy of the molecules and make them more likely to react with each other. On the other hand, decreasing the temperature would decrease the rate of the reaction since it would decrease the kinetic energy of the molecules and make them less likely to react with each other.Adding a catalyst would also increase the rate of the reaction since it would lower the activation energy required for the reaction to occur. Therefore, the answer to the question is that all the options listed would affect the rate of a chemical reaction, and none of them would be expected to have no effect on the reaction.
learn more about chemical reaction Refer: https://brainly.com/question/29762834
#SPJ11
A geologist is making observations from atop a small mountain. She sees two parallel faults: one directly to the east and one directly to the west. She concludes that these faults are caused by tension stretching the crust. From what type of land feature is the geologist making her observations? plateau anticline syncline fault-block mountain
Answers
Answer: fault-block mountain
Explanation:
I got it right on edgen 2020
Answer:
d fault-block mountain
Explanation: