The nucleus of a fluorine atom has a charge of?
A. +19
B. 0
C. +9
D. +1
Answers
Answer: B
Explanation: A fluorine atom has nine protons and nine electrons, so it is electrically neutral. (neutral =0)
The nucleus of a fluorine atom has a charge of zero as the atom has equal number of positive and negative charges.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/29695801
#SPJ6
Related Questions
HELPPPP !!! oxygen calculation to find neutrons
Answers
Answer:
Oxygen has 8 neutrons
Explanation:
We know that cause the atomic number says how many protons does the element has, so in this case is eight, and in the mass number, we have the number of protons+neutrons, in this case is 16. Then, as we know that oxygen has eight protons with the atomic number, we would do 16-8 protons= 8 neutrons.
Check: Atomic number is 16= 8 protons + 8 neutrons
Avogadro's number is the number of particles in one mole of a pure substance.
Answers
Avogadro's number is approximately 6.022 x 10^23, representing the number of particles in one mole of a substance.
Avogadro's number, denoted as N<sub>A</sub>, is a fundamental constant in chemistry and physics. It represents the number of particles, specifically atoms or molecules, in one mole of a pure substance. The value of Avogadro's number is approximately 6.022 x 10<sup>23</sup> particles per mole.
The concept of Avogadro's number is essential for understanding the relationship between the macroscopic and microscopic worlds.
It allows scientists to bridge the gap between measurable quantities, such as mass or volume, and the atomic or molecular scale. One mole of any substance contains Avogadro's number of particles, regardless of the element or compound.
Avogadro's number enables calculations involving the mole, such as determining the number of atoms or molecules in a given sample, or converting between mass and moles.
It is a cornerstone of stoichiometry, the branch of chemistry concerned with the quantitative relationships between reactants and products in chemical reactions.
In summary, Avogadro's number is a crucial constant that facilitates understanding and calculations involving the vast number of particles present in one mole of a pure substance.
Learn more about Moles
brainly.com/question/15209553
#SPJ11
Predict the product for the reaction of R-2-chlorobutane with NaI in acetone, indicating correct stereochemistry.
Answers
The substrate is R-2-chlorobutane, the nucleophile will attack from the opposite side of the leaving group to give an inversion of configuration at the chiral center. Thus, the product will be S-2-iodobutane.
The reaction of R-2-chlorobutane with NaI in acetone is an example of an SN2 nucleophilic substitution reaction. In this reaction, the iodide ion (I-) acts as a nucleophile, attacking the carbon atom that is attached to the chlorine atom in R-2-chlorobutane.
The stereochemistry of the product will depend on whether the nucleophile attacks from the same side or the opposite side of the leaving group (chlorine) in the substrate.
The reaction can be represented by the following equation:
R-2-chlorobutane + NaI → S-2-iodobutane + NaCl
The product S-2-iodobutane has a chiral center at the second carbon atom, and the iodine atom is attached to the opposite side (i.e., the S-side) of the chlorine atom that was originally attached to the substrate.
Here you can learn more about S-2-iodobutane
https://brainly.com/question/31436020#
#SPJ11
2.
Use electron configurations to explain the bonding between lithium and chlorine. Be sure to
include the formula for lithium chloride.
Answers
Explanation:
Electronic Configuration for Li: 1s²2s¹
Electronic Configuration for Cl: [Ne]3s²3p⁵
When Lithium and Chlorine bonds together, the electron in the Lithium 2s subshell will be transfered to the Chlorine 3p subshell, giving both the Lithium and Chlorine atoms a complete 1st and 3rd energy levels respectively.
Formula for Lithium chloride: LiCl.
Which of the following statements is true?
A.Most meteors fall into Earth's oceans, where they cause no damage.
B.Jupiter is the only planet which rotates almost on its side.
C.Earth's blue color is the result of methane in the atmosphere.
D.Scientists believe that Mars once had water on its surface.
Answers
Answer:
d.
Explanation:
Answer:
D!
Explanation:
A is not true! When big meteors fall into the ocean, they cause mega-tsunamis I think, which does a lot of damage.
B, no, Uranus does too.
C, I think this is untrue, red wavelength and reflects the blue the blue!
D, is so true! A lot of reliable sources on this one!
Sorry if I did any wrong, I haven't learn chemistry in school yet.
A total of 75.0 joules of heat are absorbed as 3.5 moles of lead are heated from 12.0°C to 52.0°C. From this data, what is the specific heat of lead? Round to the nearest thousand
Answers
A total of 75.0 joules of heat are absorbed as 3.5 moles of lead are heated from 12.0°C to 52.0°C.The specific heat of lead is 142.8× 10²³ J/gºC.
What is specific heat ?The term specific heat is defined as the quantity of heat required to increase the temperature of one gram of a substance by one Celsius degree. Its units are J/(kg K) or J/(kg °C).
Given:
Q = m × c ×ΔT
T = 52.0°C - 12.0°C
= 40°C
M = 21 × 10²³grams
Q = 75.0 joules
C = Q / M × ΔT
C = 75 J /21 × 10²³grams × 40.0
C = 3.5 × 10-²³ × 40× 10²³
C = 142.8× 10²³ J/gºC
Thus, the specific heat of lead is 142.8× 10²³ J/gºC.
To learn more about the specific heat, follow the link;
https://brainly.com/question/11297584
#SPJ1
what are the features of a standard hydrogen electrode? a temperature of 298 k a carbon electrode hydrogen gas at 1.01 x 10^5 pa (1 atm) pressure
Answers
The features of a standard hydrogen electrode are :
1. Temperature of 298 K (25°C)
2. Carbon electrode
3. Hydrogen gas at 1.01 x 10^5 Pa (1 atm) pressure
4. Electrolyte solution containing a hydrogen ion activity of 1 mol/L
5. Platinum wire as the current collector
6. A Potential of 0.00 V (relative to the hydrogen gas)
These features are what make up the Standard Hydrogen Electrode (SHE). The temperature of 298 K is the temperature at which the SHE is calibrated and is the standard temperature used in most laboratory experiments. The carbon electrode serves as the interface between the hydrogen gas and the electrolyte, and the hydrogen gas is held at 1.01 x 10^5 Pa (1 atm) pressure. The electrolyte solution contains a hydrogen ion activity of 1 mol/L, which is necessary for the electrode to function properly. A platinum wire is used as the current collector, and the electrode has a potential of 0.00 V, relative to the hydrogen gas. All of these features are necessary for the SHE to function properly and for the electrode to serve as the reference for all other electrochemical measurements.
To know more about electrodes refer to the link brainly.com/question/17060277
#SPJ4
Which of these ions is most likely to be leached from the soil?A) magnesium ions,B) chlorine ions,C) calcium ions,D) Iron ions, orE) potassium ions
Answers
The ions that is most likely to be leached from the soil is the correct option is B) chlorine ions.
The Chlorine ions are the negatively charged ions and are this is the reason it is not likely to be bound to the negatively charged soil particles. The Chlorine kills most of the microbes in the soil. Making the worm tea with the chlorinated water that defeats the purpose of the worm tea.
The Leaching occurs as the excess water removes the water - soluble nutrients out of the soil, by the runoff or the drainage. The Leaching is the environmental problem for the agricultural professionals.
To learn more about chlorine here
https://brainly.com/question/13346081
#SPJ4
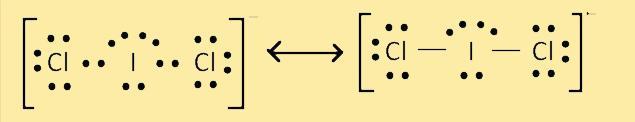
What is the number of lone pairs on the central atom in the lewis structure of icl2- ?
Answers
The central atom of ICl2- has three lone pairs in the Lewis structure.
Clarify the Lewis structure.Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs), are diagrams that show the covalent bonds that connect atoms in a molecule as well as any lone electron pairs that may be present. Any covalently bonded molecule, as well as coordination compounds, can be given a Lewis structure. Gilbert N. Lewis, who proposed the Lewis structure in his 1916 article The Atom and the Molecule, was named after it. Lewis structures extend the concept of the electron dot diagram by drawing lines between atoms to represent shared pairs in a chemical bond.
Learn more about Lewis electron dot structures here:-
https://brainly.com/question/11967426
#SPJ4
Zinc has a density of 7.10 g/cm3. If a cylinder of zinc weighing 46.77 g is completely immersed in a graduated cylinder that originally contains 33.33 mL of water, what will be the new water level? PLEASE HELP!!!! I NEED TO TURN THIS IN SOON!
Answers
New water level = 33.33+ 6.6 = 39.93 ml
Can you mark it brainliest if my answer is correct?
The Density of a substance is simply defined as the mass of the subtance per unit volume of the substance i.e
Density = mass /volume
To solve the question given above, we'll begin by calculating the volume of the zinc. This can be obtained as follow:
Density of Zinc = 7.10 g/cm³
Mass of Zinc = 46.77 g
Volume of Zinc =?Density = mass /volume
7.10 = 46.77 / Volume
Cross multiply
7.10 × Volume = 46.77
Divide both side by 7.10
Volume = 46.77 / 7.10
Volume of Zinc = 6.59 cm³Finally, we shall determine the new volume of the water. This can be obtained as follow:
Volume of Zinc = 6.59 cm³ = 6.59 mL
Volume of water = 33.33 mL
New volume of water =?New volume of water = (Volume of Zinc) + (Volume of water)
New volume of water = 6.59 + 33.33
New volume of water = 39.92 mLTherefore, the new volume of water is 39.92 mL
Learn more: https://brainly.com/question/24472494
An ice hockey puck travels 18m in 3s before it slides into the goal.What is the speed of the traveling puck?
Answers
Answer: 6m/s
Explanation:
Speed is calculated as distance divided by time. Therefore, the speed of the traveling puck would be calculated as the distance travelled by the hockey puck divided by the time taken. This will be:
= 18m / 3s
= 6m/s
The answer is 6 meters per second
Explain how the
vegetation in our area changes with our seasons.
Answers
Answer:
it changes when you put water on it
Explain why an orange is the color orange.
Answers
Answer:
Because
Explanation:
A orange is called a orange because it is the color orange
as well as orange is orange because of the orange
You have a reaction: A + B -->C The reaction is first order with respect to A and 2nd order with respect to B. If 0.6892 mol of A is mixed with 0.8342 mol of B to make a 1.000 L solution and the rate of the reaction is 0.1952 M/s, what is the rate constant (k)? Do NOT INCLUDE Units QUESTION 3 1 points Save Answer Which factor does not affect the kinetics of a reaction? O enthalpy of reaction reactant concentration O tempearture O catalyst
Answers
The rate constant (k) for the given reaction is 0.3695 mol⁻².L².s⁻¹.
The correct option for the second question is enthalpy of reaction.
Reaction is a process where reactants undergo a transformation and lead to the formation of products. The factors that affect the kinetics of a reaction are temperature, catalyst, concentration, surface area and pressure. Out of these, enthalpy of reaction is not a factor affecting the kinetics of a reaction.
A given reaction is first order with respect to A and second order with respect to B. The rate law for the reaction is given as-
Rate = k[A][B]²
Given data- [A] = 0.6892 mol; [B] = 0.8342 mol; rate = 0.1952 M/s
We know that, for any chemical reaction, the rate constant (k) is the proportionality constant between the rate of the reaction and the concentrations of the reactants. Using the given values, we can substitute them in the rate law and calculate the value of the rate constant (k).0.1952 M/s = k (0.6892 M) (0.8342 M)²k = 0.3695 mol⁻².L².s⁻¹
Therefore, the rate constant (k) is 0.3695 mol⁻².L².s⁻¹.
The correct option is enthalpy of reaction.
To learn more about "rate constant", visit: https://brainly.com/question/24749252
#SPJ11
Describe how you would prepare a pure dry sample of lead(II) sulfate crystals starting from solutions of lead(II) nitrate and sodium sulfate.
Include a series of key steps in your answer.
Answers
Answer:
Method: Measure out 25 cm3 of 0.5 mol dm3 lead(II)nitrate solution and add it to a small beaker. Measure out 25 cm3 of 0.5 mol dm3 of potassium sulfate add it to the beaker and mix together using a stirring rod.
Please hurry!!
Decide if this model shows
1. covalent or ionic and what evidence for your decision 4 pts
2. What type of element Are Y and Z evidence 4 pts
Bonus Name the compound using two of these fruit names
metal apple, nonmetal grape, nonmetal pineapple

Answers
The given model shows the covalent bond.
A covalent bond includes the mutual sharing of one or more pairs of electrons among atoms. these electrons are concurrently attracted via the 2 atomic nuclei. A covalent bond bureaucracy when the difference among the electronegativities of atoms is just too small for an electron transfer to arise to form ions.
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. these electron pairs are referred to as shared pairs or bonding pairs. The stable stability of appealing and repulsive forces between atoms, once they share electrons, is known as covalent bonding.
Learn more about covalent bonds here:-https://brainly.com/question/3447218
#SPJ1
How was Bohr’s atomic model different from Rutherford’s atomic model?
Answers
Answer:
Bohr thought that electrons orbited the nucleus in circular paths; whereas in the modern view atomic electron structure is more like 3D standing waves. ... He believed that electrons moved around the nucleus in circular orbits with quantised potential and kinetic energies.
Answer:
The answer is A electrons exist in specified energy levels surrounding the nucleus. for people in edguinity
Explanation:
A cylinder shaped water tank has a height of 212 ft (6461.76 cm), and is able
to hold 15,000 gallons of water (15,000,000 mL or 15,000,000 cm3). The
volume of a cylinder is expressed by the equation
V = tr^2h
Where V is the volume, r is the radius, and h is the height of the cylinder.
Express the radius (r) in terms of volume (V) and height (h), then determine
the radius of a cylinder water tank (in centimeters) that is filled to capacity.

Answers
Answer:
27.19 cm.
Explanation:
From the question given above, the following data were obtained:
Height (h) of cylinder = 6461.76 cm
Volume (V) of cylinder = 15000000 cm³
P1 (π) = 3.14
Radius (r) =.?
Thus, we can obtain the radius of the cylinder water tank by using the following formula as illustrated below:
V = πr²h
15000000 = 3.14 × r² × 6461.76
15000000 = 3.14 × 6461.76 × r²
Divide both side by 3.14 × 6461.76
r² = 15000000 / (3.14 × 6461.76)
Take the square root of both side
r = √[15000000 / (3.14 × 6461.76)]
r = 27.19 cm
Thus, the radius of the cylinder water tank is 27.19 cm.
Why are ethics important when considering experiments?
A. A general sense of ethics ensures that everyone is aware of what's
right and what's wrong.
B. The scientific results would not be believable if they were obtained
unethically
C. Scientists would not be able to get funding if their experiments
were not ethical
D. It is important that people are not harmed for the sake of science,
Answers
Answer:
C. is the correct answer
Explanation:
I took the test
75.0 g sample of a metal at 65°C is added to 100.0 g of water at 20.0°C. The temperature of the water rises to 22.3 °C. Calculate the specific heat of the metal, assuming that all the heat lost by the metal is gained by the water.
Answers
The specific heat of the metal is 0.30J/g°C.
What is specific heat capacity?The specific heat capacity is defined as the quantity of heat (J) absorbed per unit mass (kg) of the material when its temperature increases 1 K (or 1 °C), and its units are J/(kg K) or J/(kg °C).
Step 1: Data given
Mass of metal = 75.0 g
Temperature of the metal = 65°C
Mass of water = 100.0 g
Temperature of the water = 20.0°C
Final temperature of the water = 22.3 °C
The specific heat of water is 4.184 J/g°C
Step 2: Calculate the specific heat of metal
Qlost = -Qgained
Q= m xCxΔT
Qmetal = -Qwater
m(metal) x c(metal) x ΔT(metal) = - m(water) x c(water) x ΔT(water
⇒m(metal) = the mass of metal = 75.0 g
⇒c(metal) = the specific heat of the metal = ?
⇒ΔT = The change of temperature = 22.3 - 65°C = -42.7 °C
⇒m(water) = the mass of water = 100.0 g grams
⇒c(water) = the specific heat of water = 4.186 J/g°C
⇒ΔT(water) = T2 - T1 = 22.3 - 20.0 = 2.3 °C
75.0 g x c(metal) x -42.7 °C= -100.0 g x 4.186 x 2.3
75.0 g x c(metal) x -42.7 °C = −962.78
c(metal) = 0.3006338798J/g°C =0.30J/g°C
The specific heat of the metal is 0.30J/g°C.
Learn more about specific heat capacity here:
https://brainly.com/question/1747943
#SPJ1
What is the ratio between the maximum and the minimum sound intensities that produce this particular loudness?
Answers
The ratio between the maximum and minimum sound intensities that produce a particular loudness is known as the dynamic range.
The specific ratio can vary depending on the loudness level and the individual's perception.For example, the dynamic range for a loudness level of 60 dB may be around 1,000 to 1, meaning the maximum sound intensity is 1,000 times louder than the minimum sound intensity required to produce that loudness level.
However, in general, the dynamic range for human hearing is estimated to be around 120 decibels, meaning that the loudest sound we can tolerate is around 120 dB greater than the quietest sound we can hear.
To know more about dynamic range click on below link :
https://brainly.com/question/30481254#
#SPJ11
why natural fas is not used as a bottled gas or as a motor fuel?
Answers
Answer:
Natural gas is an odorless, gaseous mixture of hydrocarbons—predominantly made up of methane (CH4). It accounts for about 30% of the energy used in the United States. About 40% of the fuel goes to electric power production and the remaining is split between residential and commercial uses, such as heating and cooking, and industrial uses. Although natural gas is a proven, reliable alternative fuel that has long been used to power natural gas vehicles, only about two-tenths of 1% is used for transportation fuel.
The vast majority of natural gas in the United States is considered a fossil fuel because it is made from sources formed over millions of years by the action of heat and pressure on organic materials. Alternatively, renewable natural gas (RNG), also known as biomethane, is a pipeline-quality vehicle fuel produced from organic materials—such as waste from landfills and livestock—through anaerobic digestion. RNG qualifies as an advanced biofuel under the Renewable Fuel Standard.
Because RNG is chemically identical to fossil-derived conventional natural gas, it can use the existing natural gas distribution system and must be compressed or liquefied for use in vehicles.
CNG and LNG as Alternative Transportation Fuels
Two forms of natural gas are currently used in vehicles: compressed natural gas (CNG) and liquefied natural gas (LNG). Both are domestically produced, relatively low priced, and commercially available. Considered alternative fuels under the Energy Policy Act of 1992, CNG and LNG are sold in units of gasoline or diesel gallon equivalents (GGEs or DGEs) based on the energy content of a gallon of gasoline or diesel fuel.
Compressed Natural Gas
CNG is produced by compressing natural gas to less than 1% of its volume at standard atmospheric pressure. To provide adequate driving range, CNG is stored onboard a vehicle in a compressed gaseous state at a pressure of up to 3,600 pounds per square inch.
CNG is used in light-, medium-, and heavy-duty applications. A CNG-powered vehicle gets about the same fuel economy as a conventional gasoline vehicle on a GGE basis. One GGE equals about 5.66 pounds of CNG.
Liquefied Natural Gas
LNG is natural gas in its liquid form. LNG is produced by purifying natural gas and super-cooling it to -260°F to turn it into a liquid. During the process known as liquefaction, natural gas is cooled below its boiling point, removing most of the extraneous compounds found in the fuel. The remaining natural gas is primarily methane with small amounts of other hydrocarbons.
Because of LNG's relatively high production cost, as well as the need to store it in expensive cryogenic tanks, the fuel's widespread use in commercial applications has been limited. LNG must be kept at cold temperatures and is stored in double-walled, vacuum-insulated pressure vessels. LNG is suitable for trucks that require longer ranges because liquid is denser than gas and, therefore, more energy can be stored by volume. LNG is typically used in medium- and heavy-duty vehicles. One GGE equals about 1.5 gallons of LNG.
Aplant whose seeds are exposed rather than protected by fruit is called sli)
A plant that has Neers and whose seeds are protected by fruit is called sly)

Answers
Answer:
Ik Gymnosperms
Explanation:
term that means naked seeds
Answer:
gymnosperm and angiosperm
Explanation:
How much energy is needed to convert 15.0 g of ice at -5.0 oC into water at 50.0 oC?
Answers
Using thermodynamics
\(\\ \sf\longmapsto Q=mc∆T\)
\(\\ \sf\longmapsto Q=0.015(328)(4184)\)
\(\\ \sf\longmapsto Q=20585.28J\)
The disinfectant hydrogen peroxide (H2O2)
decomposes to form water and oxygen gas.
How much O2 will result from the decomposition of 2.22 mol of hydrogen peroxide?
Answers
Answer:
1.1 mol of O2
Explanation:
First we need the balance chemical equation which is
2 H2O2 -------> 2 H2O + O2
This is important because in stoichiometry you can go from units of one thing to other by using mole ratios, here the mole ratio is 2 mol of H2O2 for one mole of O2.
\(2.2 mol H2O2 \frac{1mol O2}{2 mol H2O2}\) = 1.1 mol of O2
What is gesso? a. A technique used by many Renaissance artists b. A medium used as a surface preparation or primer for a painting c. A mixture of egg yolk and pigment used to harden paint.
Answers
Gesso is a white paint blend that comprises pigment, chalk and gypsum. It is a medium utilised for surface preparation and as a primer for a painting.
What are the uses of gesso?Gesso is widely utilized for preparing surfaces and as primers by painters. They were used by painters and artists earlier to make canvas, boards for painting purposes.
It was first formulated by chalk powder dust and pigment derived from the animal skin. They are widely used because of their adhering properties.
The use of the gesso makes the paint adhere to the canvas and the board surfaces and also, makes the surface textured.
Therefore, option b. it is used for preparing the surface is correct.
Learn more about gesso here:
https://brainly.com/question/1191408
Answer:
b on edge
Explanation:
an established turf that is too acidic can be effectively topdressed with agricultural lime to lower ph. True/False ?
Answers
It is true that an established turf that is too acidic can be effectively top dressed with agricultural lime to lower ph.
pH stands for "potential hydrogen" and refers to the measure of acidity or alkalinity of a solution. pH is a scale that ranges from 0 to 14, where 0 is highly acidic, 7 is neutral, and 14 is highly alkaline. The pH scale is logarithmic, meaning that a solution with a pH of 6 is ten times more acidic than a solution with a pH of 7. The pH of a solution is important because it affects the solubility and availability of nutrients in soil for plants, and it can also impact the growth and survival of aquatic organisms in water. Maintaining the proper pH level is essential for many biological systems and can help ensure optimal growth and health.
Learn more about ph:
brainly.com/question/2288405
#SPJ4
How much energy is required to raise the temperature of 3 kg of lead from 15°C to 20°C? Use the table below and this equation: Q = MCAT.
The question is written right above the table given.
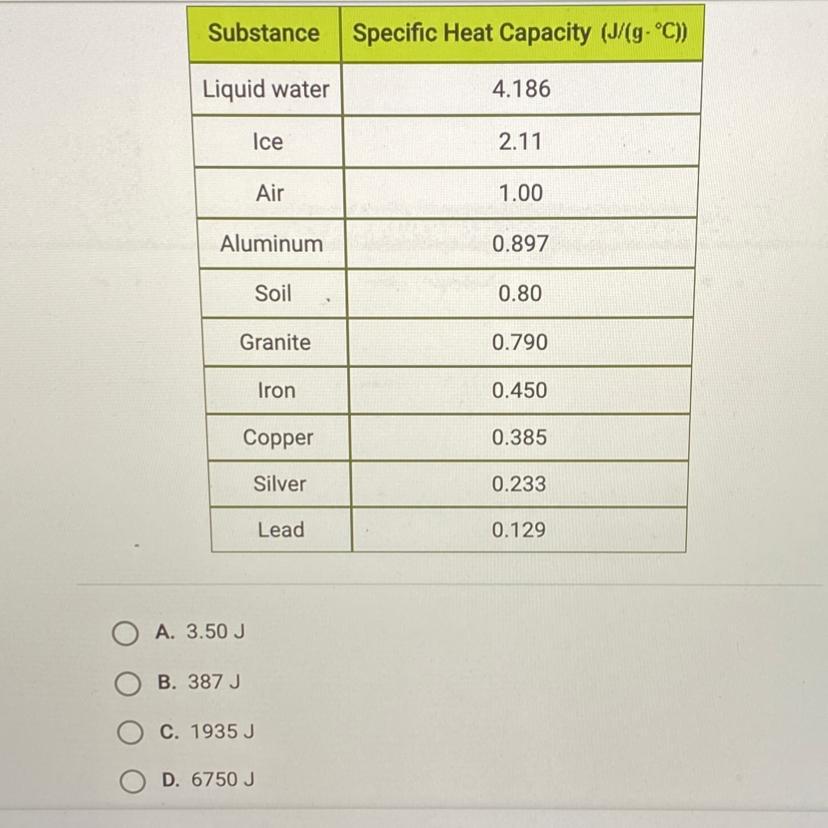
Answers
Answer:
1935J
Explanation:

Answer:
\(\boxed {\boxed {\sf C. \ 1935 \ J}}\)
Explanation:
The equation for this problem is:
\(q=mc\Delta T\)
where m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
The mass is 3 kilograms, but the specific heat capacity includes grams in the units. Convert kilograms to grams. There are 1000 grams in 1 kilogram.
\(\frac {1000 \ g}{1 \ kg}\)\(3 \ kg *\frac {1000 \ g}{1 \ kg}\)\(3 *1000 \ g = 3000 \ g\)The specific heat capacity for lead is found on the table. It is 0.129 J/g°C.
Let's find the change in temperature. It is raised from 15 °C to 20 °C.
\(\Delta T= final \ temperature - initial \ temperature \\\Delta T= 20 \textdegree C - 15 \textdegree C\\\Delta T= 5 \textdegree C\)Now we know every value.
m= 3000 g c= 0.129 J/g°CΔT= 5 °CSubstitute the values into the formula.
\(q= (3000 \ g)( 0.129 \ J/g \textdegree C)(5 \textdegree C)\)
Multiply the first 2 numbers together. The units of grams cancel.
\(q= (387 \ J/ \textdegree C )(5 \textdegree C)\)
Multiply again. This time the units of degrees Celsius cancel.
\(q= 1935 \ J\)
1935 Joules of energy are required and choice C is correct.
Las prótesis, válvulas del corazón, marca pasos, clavos en problemas de huesos quebrados, son productos que evolucionaron en materiales para ser más amigables con el cuerpo humano y que no causen rechazo y de esa manera contribuir a prolongar la vida de calidad de una persona, esta evolución se debe al desarrollo de nuevos materiales en el campo de los…
Answers
Answer:
porh hun xxxx ysn po answet
1)Why do clouds usually form high in the air instead of near Earth's surface?
a) High in the atmosphere is less dense
b) High in the atmosphere contains less oxygen
c) High in the atmosphere is usually cooler
d) High in the atmosphere has less air pressure