the general chemical composition of various window glasses has been found to be relatively uniform among various manufacturers and therefore offers no basis for identifying individual samples.truefalse
Answers
The statement "the general chemical composition of various window glasses has been found to be relatively uniform among various manufacturers and therefore offers no basis for identifying individual samples" is True.
Glass is a commonly used material that is used in a wide range of applications, including window panes. The chemical composition of window glass is relatively constant among different manufacturers, according to research. Therefore, it does not provide a reliable method for identifying various glass samples based on their chemical composition.
The given statement "the general chemical composition of various window glasses has been found to be relatively uniform among various manufacturers and therefore offers no basis for identifying individual samples" is true, because the chemical composition of glass is constant, researchers employ alternative techniques to distinguish between various glass samples. Some of these techniques include the use of trace elements, the age of the glass, and other physical characteristics such as refractive index and density.
Therefore the given statement is true.
Learn more about glass on:
https://brainly.com/question/465042
#SPJ11
Related Questions
Balancing Chemical Equation
CuCl2+H2S=CuS+HCl
Answers
Answer:
CuCi2 + H2S ⇒ CuS + 2HCi
Explanation:
In order to balance a chemical equation you need to make sure to have the same number of atoms on each side by multiplying on both sides.
CuCi2 + H2S = CuS + HCi
Cu = 1
Ci = 2
H = 2
S = 1
Product
Cu = 1
S = 1
H = 1 × 2 = 2
Ci = 1 × 2 = 2
Since all elements have a balanced amount of atoms, the equation is now balanced.
CuCi2 + H2S ⇒ CuS + 2HCi
Hope this helps.
A chemical equation is said to be balanced if the quantity of each type of atom in the reaction is the same on both the reactant and product sides. In a balanced chemical equation, the mass and the charge are both equal. Here the balanced equation is CuCl₂ + H₂S → CuS + 2HCl.
A balanced chemical equation, in which the masses of the reactants and products are equal, contains the same amount of atoms of each element on both sides of the equation. In other words, both sides of the reaction have an equal balance of mass and charge.
The chemical equation is said to be balanced if there are no inequalities. Here the balanced equation is:
CuCl₂ + H₂S → CuS + 2HCl
To know more about balanced equation, visit;
https://brainly.com/question/14812568
#SPJ6
How can we determine whether a ubtance i acidic or baic uing the filter paper and the pH trip?
Answers
To determine whether a substance is acidic or basic using filter paper and a pH indicator, you can follow these steps:
Obtain a filter paper and moisten it with a small amount of the substance in question; Touch the filter paper with a pH indicator, such as litmus paper or universal indicator; Observe the color change of the pH indicator in contact with the substance; Compare the color change with the reference chart for the pH indicator being used to determine the pH of the substance.
If the substance turns the pH indicator blue, it is basic (alkaline) with a pH greater than 7. If the substance turns the pH indicator red, it is acidic with a pH less than 7. If the substance does not change the color of the pH indicator, it is neutral with a pH of 7.
Note: The pH scale ranges from 0 to 14, with 7 being neutral, less than 7 being acidic, and greater than 7 being basic.
To know more about pH please refer:
https://brainly.com/question/15289741
#SPJ4
chloride per milliliter (MW of CaCl2 = 147) [Round to the nearest whole number 5. What weight of magnesium chloride (MgCl2, formula weight = 95.3) is required to prepare 200 ml solution that is 5.0 mi
Answers
The weight of magnesium chloride required to prepare the 200 ml solution that is 5.0 M is approximately 48 grams.
To calculate the weight of magnesium chloride (\(MgCl_{2}\)) required to prepare a 200 ml solution that is 5.0 M, we need to use the formula: Weight (in grams) = Volume (in liters) × Concentration (in moles/liter) × Molecular Weight (in grams/mole)
First, we convert the volume from milliliters to liters by dividing it by 1000: Volume = 200 ml ÷ 1000 = 0.2 L. Next, we multiply the volume, concentration, and molecular weight: Weight = 0.2 L × 5.0 mol/L × 95.3 g/mol = 47.65 grams
Rounding to the nearest whole number, the weight of magnesium chloride required to prepare the 200 ml solution that is 5.0 M is approximately 48 grams.
This calculation ensures that the desired concentration is achieved by accurately measuring the appropriate amount of magnesium chloride, taking into account its molecular weight and the desired volume of the solution.
To know more about magnesium chloride, refer here:
https://brainly.com/question/30671024#
#SPJ11
someone help me please....
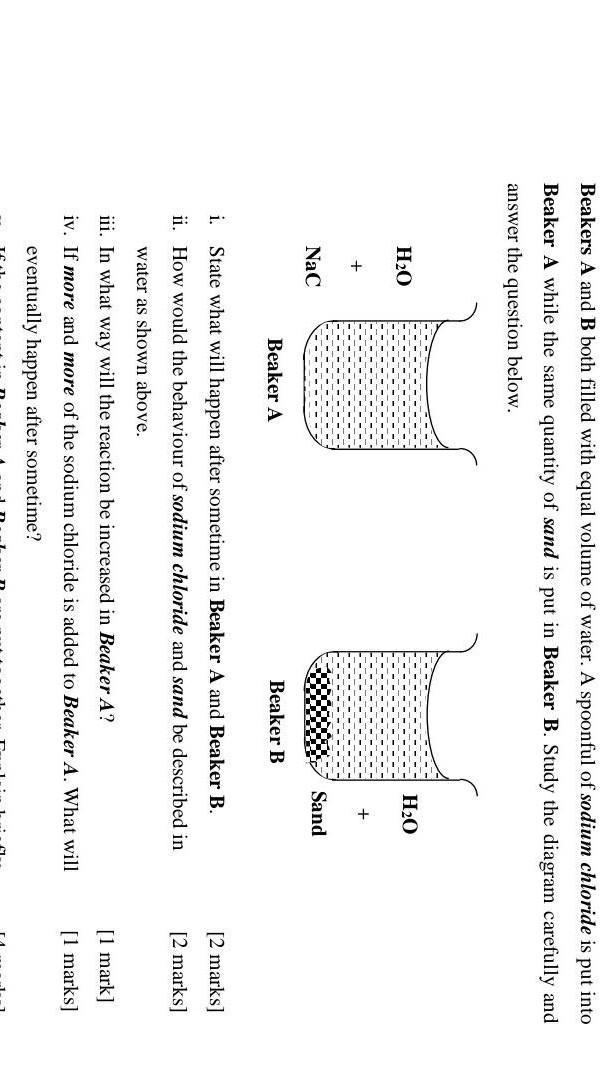
Answers
Answer:
i- In beaker A, sodium chloride will dissolve with water.
and in beaker B, there will be no reaction.
ii- sodium chloride is soluble in water, while sand is insoluble.
iii- The reaction can be increased by adding more sodium chloride to the beaker.
iv- The sodium chloride will no longer dissolve in the water.
According to the electron sea model, the melting points of metals are not as extreme as the boiling points. This is because the cations and electrons are - in a metal. It does not take much energy for a solid to become liquid. But metallic bonds are very - , so it does require a lot of energy to separate atoms from the cations in their sea of electrons.
Answers
According to the electron sea model, the melting points of metals are not as extreme as the boiling points. Therefore, the given statement is correct is true.
What is electron sea model?The Electron Sea Model's whole hypothesis relies around the behavior of atoms throughout this bonding. The movement of unpaired electrons between positively charged metal ions in a mesh is known as metallic bonding.
According to the electron sea model, the melting points of metals are not as extreme as the boiling points. This is because the cations and electrons are - in a metal. It does not take much energy for a solid to become liquid. But metallic bonds are very - , so it does require a lot of energy to separate atoms from the cations in their sea of electrons. This statement is true.
Therefore, the given statement is correct is true.
To know more about electron sea model, here:
https://brainly.com/question/30003484
#SPJ1
Scientists are developing new methods of conservation to preserve species in danger of extinction. Captive breeding programs try to restore the population of a species. Germ plasm is any form of genetic material contained in reproductive or germ cells or plants and animals. Germ plasm banks also knowns as gene banks save sperm, egg or DNA. Farmers also save germ seeds to replant seeds of plants.
13. One way scientists save species in danger of extinction is by:
A logging tropical rain forests
B developing new methods of conservation
C removing species from endangered lists
D abandoning new methods of conservation
Answers
Answer:
B. Developing new methods of conservation
Explanation:
Such as the Germ Plasm in the excerpt. This is an example of new conservation methodology
What is the molarity of a solution prepared with 78.90g of calcium chloride in 750.0 mL of water?
Answers
Step 1
Molarity is a type of concentration and it is defined as:
\(Molarity\text{ \lparen M or mol/L\rparen = }\frac{Moles\text{ of solute \lparen CaCl2\rparen}}{Volume\text{ of the solution \lparen L\rparen}}\text{ \lparen1\rparen}\)---------------------
Step 2
Information provided:
78.90 g calcium chloride (CaCl2)
750.0 mL of water => it is assumed that the volume of water is equal to the volume of solution.
750.0 mL x (1 L/1000 mL) = 0.7500 L
-----
Information needed:
The molar mass of CaCl2 = 111 g/mol approx. (please, use your periodic table)
-----------------------
Step 3
Procedure:
Moles of CaCl2 = mass/molar mass = 78.90 g/111 g/mol = 0.710 moles
----
Molarity from (1):
Molarity = 0.710 moles/0.7500 L = 0.95 mol/L or M
Answer: Molarity = 0.95 mol/L
What MASS of NaCl are required to make 2.69L of a 0.14M solution?Use the correct abbreviation for the UNITS
Answers
To solve this problem, let's use the definition for molarity:
Replacing the values of the problem:
Now, to find the mass, we multiply by the molecular weight of NaCl. (Which is about 58.44g/mol)
The answer is approximately 22.2g of NaCl



Please help me with those questions please

Answers
Answer:
I know the answer to 5, i think
Explanation:
No, if both parents were left-handed, they would not have to be left-handedSelect all the correct answers.
Which two statements are true about energy transformations?
Energy is never released from an object as heat.
Energy is never transformed between kinetic energy and potential energy.
O Energy is never created.
Energy is never completely transformed.
Energy is never destroyed.
Reset
Next
Answers
True Statements
Energy is never destroyedEnergy is never completely transformedEnergy is never createdFalse Statements
Energy is never released from an object as heatEnergy is never transformed between kinetic energy and potential energySe ard 25g de magneziu de puritate 90%. Ce volum de oxigen se consumă și câti moli dioxid de magneziu se formează
Answers
Answer:
10.5 dm3 O2
0.94 moles de MgO
Explanation:
La pregunta dice explícitamente que el magnesio es puro en un 90%.
Por lo tanto, masa de magnesio puro = 90/100 * 25G = 22,5 g
Número de moles de Mg = 22,5g / 24g/mol = 0,94 moles de Mg
La ecuación de reacción es;
2Mg (s) + O2 (g) ------> 2MgO (s)
Si 2 moles de Mg reaccionan con 1 moles de O2
0.94 moles de Mg reacciona con 0.94 * 1/2 = 0.47 moles de O2
Si 1 mol de O2 ocupa 22,4 dm3
0.47 moles de O2 ocupan 0.47 * 22.4 / 1 = 10.5 dm3
También;
2 moles de Mg producen 2 moles de MgO
Entonces, 0.94 moles de Mg producen 0.94 moles de MgO
Se sabe que 10 g de calcio reaccionan con 4 g de oxígeno para obtener 14 g de óxido de calcio. Indica la cantidad de óxido de calcio que se obtiene al hacer reaccionar cantidades iguales de calcio y oxígeno (por ejemplo, 50 g de cada uno)
Answers
Answer:
Si se usan 50 gramos de calcio y óxigeno, se obtienen 70 gramos de óxido de calcio.
Explanation:
Hola,
En este caso, la reacción llevada a cabo es:
\(2Ca+O_2\rightarrow 2CaO\)
De este modo si asumimos el ejemplo dado, 50 gramos de calcio, cuya masa atómica es 40 g/mol y 50 g de oxígeno, cuya masa atómica como gas diatómico es 32 g/mol, antes de calcular los gramos de óxido de calcio producidos, debemos identificar el reactivo límite. Así, calculamos las moles de calcio disponibles en 50 g:
\(mol_{Ca}^{disponible}=50gCa*\frac{1molCa}{40gCa} =1.25molCa\)
Y también las moles de calcio consumidas por los 50 g de oxígeno, utilizando su relación molar 2:1:
\(mol_{Ca}^{consumidas\ por\ O_2}=50gO_2*\frac{1molO_2}{32gO_2} *\frac{2molCa}{1molO_2} =3.125molCa\)
Por lo tanto, hay menos calcio disponible que el que consume el oxígeno, por lo que el calcio esel reactivo límite. Ahora, con este, calculamos los gramos de óxido de calcio, cuya masa molar es 56 g/mol, que se producen:
\(m_{CaO}=1.25molCa*\frac{2molCaO}{2molCa}* \frac{56gCaO}{1molCaO}\\ \\m_{CaO}=70gCaO\)
Esto quiere decir que de 50 gramos de oxígeno, solo 20 gramos reaccionan para formar 70 gramos de óxido de calcio.
Saludos!
Answer:
69.9 g of CaO will be produced. ≅ 70 g
Explanation:
First of all you need to make the reaction:
2Ca + O₂ → 2 CaO
Determine the moles of each reactant:
10 g Ca / 40.08 g/mol = 0.25 moles
4 g O₂ / 32 g/mol = 0.125 moles
There is no limiting reagent in this reaction, we can use both elements.
Ratio between Ca and CaO, is 2:2. For 0.25 moles of Ca I would make 0.25 moles of CaO. We convert the moles to mass:
0.25 mol . 56.08 g / 1mol = 14 g
Let's think when you have the same mass of reactant:
50 g Ca/ 40.08 g/mol = 1.24 moles
50 g O₂ / 32 g/mol = 1.56 moles
For 1 mol of oxygen I need 2 moles of calcium, so If I have 1.56 moles, I would need the double, 3.12. Notice that Ca is the limiting reagent (we need 3.12 moles of reactant, but we only have 1.24). Now we need to work with it. If 2 moles of Ca, makes 2 moles of CaO, then 1.24 moles, will produce the same amount of oxide. We finally convert the moles to mass: 1.24 mol . 56.08 g/mol = 69.9 g
for a thermometer with at every 1.0°c what is the uncertainty
Answers
For a thermometer with a mark at every 1.0°C, the uncertainty is ± 0.5°C which can result in errors.
What are errors?
Errors in chemical analysis result when there is a difference between observed value and the true value.If the magnitude of errors is large , it results in decrease in accuracy, reproducibility, and precision.
There are three types of errors:1) random error 2) systematic error 3) human error.The cause of random errors are difficult to quantify while the human errors can be minimized by taking a range of readings to reduce the error.
Learn more about errors,here:
https://brainly.com/question/15810279
#SPJ9
Suppose you are given three different solutions containing Na,PO4, Ba(NO3)2, and K,CO, respectively. Based on
the results of this lab and other reference materials, hypothesize about which combinations of these solutions
will produce insoluble precipitates. Based on your observations of the behavior of the compounds studied in this lab and in previous lessons what general statements can you make about the solubility of ionic compounds
containing Na+, Ba2+, K+, PO4-, NO3-, and CO3.
Answers
The solubility of ionic compounds depends on the nature of the ions and their charges.
Solubility of ionic compoundsIonic compounds containing Na+, K+, and NO3- ions are generally soluble in water because they have small ionic radii and weak ionic interactions. On the other hand, ionic compounds containing Ba2+, PO4-3, and CO3-2 ions tend to be less soluble in water because they have larger ionic radii and stronger ionic interactions.
Ba2+ and PO4-3 ions tend to form insoluble compounds, such as Ba3(PO4)2, while Ba2+ and CO3-2 ions can also form insoluble compounds, such as BaCO3. K+ and CO3-2 ions may also form an insoluble precipitate when combined with certain cations such as Ba2+. Overall, the solubility of ionic compounds can be influenced by factors such as temperature, pH, and the presence of other ions in the solution.
Learn more on ionic compounds here https://brainly.com/question/2687188
#SPJ1
HELP ASAP PLEASE! PLEASE
FIll in the blank

Answers
your answer will be Carbon
I just got help from a friend!
Simon measured the density of a piece of metal
to be 11.70 g/cm³. However, the manufacturer
claims it has a density of 12.00 g/cm³. What is
the percent error of Simon's measurement?
Answers
From the question we know that 12 - 11.70 = 0.3 so 0.3 / 12 = 0.025 therefore Simon's percent error is 2.5%.
Percent error compares an estimate to an accurate value and expresses the difference among them as a percent. This statistic lets analysts recognize the scale of the mistake relative to the true cost. it's also referred to as percentage error as well as % mistakes.
Percentage errors Calculation Steps :
Subtract one cost from another.Divide the mistake by the exact or ideal price (not your experimental or measured value).Convert the decimal variety into a percentage by multiplying it by using 100.Add a percent or % symbol to record your percentage mistakes value.Percentage error tells you the way huge your mistakes are when you degree something in an experiment. Smaller values suggest that you are close to the accepted or actual value. for instance, a 1% blunder means that you obtain very near the accepted value, whilst 45% means that you were pretty a long way off from the real value.
Learn more about Percent error here brainly.com/question/1600371
#SPJ1
What is a pH in biology?
Answers
Answer:
pH is a scale used to specify the acidity or basicity of an aqueous solution. Lower values correspond to solutions which are more acidic in nature, while higher values correspond to solutions which are more basic or alkaline.
Explanation:
Answer: “pH, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the hydrogen ion—which ordinarily ranges between about 1 and 10−14 gram-equivalents per litre—into numbers between 0 and 14.” - my science teacher I don’t know where she gets her notes from
Explanation:
If your NMR sample is contaminated with ethanol, where would the peaks appear (ppm values) in your 1H NMR spectrum?
Answers
If your NMR sample is contaminated with ethanol, the peaks would appear at 1.2 ppm and 3.6 ppm in your 1H NMR spectrum.
If your NMR sample is contaminated with ethanol, you will observe peaks corresponding to ethanol's protons in your 1H NMR spectrum. The peak at 1.2 ppm corresponds to the methyl group in ethanol and the peak at 3.6 ppm corresponds to the methylene group. It is important to ensure that your NMR sample is free from any contaminants to obtain accurate results. Ethanol has the chemical structure CH3CH2OH, and its peaks will appear at the following ppm values:
1. The CH3 group: This methyl group is a singlet and will appear around 1.0-1.2 ppm.
2. The CH2 group: This methylene group is a quartet and will appear around 3.6-3.7 ppm.
3. The OH group: This hydroxyl proton is a singlet and will appear in the broad range of 2.5-5.0 ppm due to hydrogen bonding and exchange effects.
Keep in mind that the exact ppm values might vary slightly depending on the solvent and experimental conditions.
Learn more about ethanol here: brainly.com/question/25002448
#SPJ11
what is the name of the addictive chemical naturally found in tobacco plants and later in tobacco products?
Answers
Answer:
Nicotine
Explanation:
How many grams are in .093 liters of O2 gas at STP?
Answers
There are 1.313 grams in .093 liters of O₂ gas at STP.
First, we need to determine the number of moles present in 0.093 liters of O₂ gas. This can be done using the ideal gas law equation which is PV = nRT where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant and T is the temperature.
We know that at STP, the temperature is 273K, the pressure is 1 atm and R = 0.0821 L atm/mol K. Therefore, n = (PV)/(RT) = (1 atm x 0.093 L)/(0.0821 L atm/mol K x 273 K) = 0.00346 mol. Next, we can find the mass of 0.00346 moles of O₂ using the molar mass of O₂ which is 32 g/mol. Therefore, mass = moles x molar mass = 0.00346 mol x 32 g/mol = 0.1107 g or 1.313 grams rounded to three significant figures.
Learn more about moles here:
https://brainly.com/question/15209553
#SPJ11
.
What is the mass, in grams, of 0.350 moles of Mg(OH)2?
Answers
Answer:
20.3 grams
Explanation:
m = n×M = 0.35×(24+(16+1)×2)= 20.3 (g)
16. You are running a TLC plate with a polar (A) and nonpolar substance (B); your solvent is a hexane and dichloromethane mixture (1:1). Substance B is traveling up the plate, but substance A has not traveled at all. What solvent might you add to the solvent mixture for substance A to move up the plate
Answers
Answer:
Acetone
Explanation:
Chromatography has to do with the separation of substances by eluting the components of the solute using a mobile phase. The composition of the mixture to be separated decides the mixture of solvents (mobile phase) that are to be used for elution.
In this case the mixture to be separated comprises of substance A which is polar and substance B which is non polar. It is observed that the mobile phase moves B up the plate but not the polar substance, A. This means that a polar solvent such as acetone should be added to the mobile phase in order to achieve better elution of A.
Quantization of energy lab Hypothesis: Make a prediction that describes the relationship between the composition of an unknown substance and its emission spectrum. For example, “If each element can be identified by its ______________, then the ____________ of an unknown star can be determined." variables: independent - dependent- control-
Answers
Answer:
If each element can be identified by its spectrum then the composition of an unknown star can be determined
Explanation:
The chemical nature of the elements is that they absorb specific wavelength of light depending on their atom. By spectral analysis of the spectrum of emitted light by a body, the body's composition can therefore be determined. As such in order to determine the composition of distant bodies such as planets, stars and other celestial bodies scientists usually make use of spectroscopy.
Answer:
If each element can be identified by its spectrum then the composition of an unknown star can be determined
Explanation:
iron corrodes because _____ turns iron into ions through the process of _____.
Answers
Iron corrodes primarily due to a process called oxidation, which turns iron into iron ions through a series of electrochemical reactions.
In the presence of water and oxygen, iron atoms lose electrons and become positively charged iron ions (Fe²⁺).
These ions then react with oxygen molecules and water to form hydrated iron(III) oxide, commonly known as rust.
This entire process is facilitated by the formation of an electrochemical cell, consisting of an anode and a cathode, on the iron surface. The anode is where oxidation occurs, while the cathode is where reduction happens.
Overall, the corrosion of iron is a natural and spontaneous process driven by the tendency of iron to revert to a more stable, oxidized state.
Learn more about oxidation at https://brainly.com/question/22815274
#SPJ11
how many O atoms are in 4C₆H₁₂O₆
Answers
Number of O atoms : 24
Further explanationGiven
C₆H₁₂O₆ compound
Required
Number of atoms
Solution
A molecular formula shows the number of atomic elements in compound.
The empirical formula is the smallest comparison of the atoms
Glucose-C₆H₁₂O₆ is composed of 3 elements, namely C, H, and O.
The number of atoms in a compound can usually be seen from the subscript number after the atom and the reaction coefficient shows the number of molecules
So number of O atoms :
= 4 x 6 = 24 atoms
Considering the stereochemistry of the inteediate I below, which of the products would you expect. Explain your answer.
Answers
The expected product is (R)-2-bromobutane.
Stereochemistry plays a crucial role in determining the outcome of chemical reactions. In the given question, the stereochemistry of the intermediate I needs to be considered to determine the expected product.
The intermediate I indicates a chiral carbon center, denoted by an asterisk (*), which means it has four different substituents attached to it. This chiral carbon results in two possible stereoisomers: (R)-2-bromobutane and (S)-2-bromobutane.
When a reaction occurs at a chiral carbon, the stereochemistry of the reactant is usually retained in the product, assuming no racemization or inversion takes place during the reaction. In this case, the intermediate I has an (R) configuration, which implies that the product will also have an (R) configuration.
Therefore, the expected product is (R)-2-bromobutane.
Learn more about (R)-2-bromobutane.
brainly.com/question/17031230
#SPJ11
Hot water is poured into four cups made of different materials (foam, glass, metal, plastic).After one minute, which will feel the warmest when you touch the OUTSIDE of the cup?
Answers
Answer:
Metal
Explanation:
Foam, glass, and plastic don't absorb as much heat as metal does.
what is a renewable resource ?
Answers
Answer:
A renewable resource is a resource that can be made, Ex: trees or energy.
Explanation:
Answer:
energy source that cannot be depleted.
Explanation:
what is an electron ?state it's relatively mass and charges.
Answers
Answer:
a stable subatomic particle with a charge of negative electricity, found in all atoms and acting as the primary carrier of electricity in solids.
mass: 9.1093837015 × 10−31 kg
charges: fundamental physical constant expressing the naturally occurring unit of electric charge, equal to 1.602176634 × 10−19 coulomb.
Explanation:
good luck !<3
1. "3D" option for Water. Why are the three circles different colors?
3. How many elements and atoms are in the compound 2Cl(CHO)2?
4.“Playground" option and make the largest molecule you can that has a name. What is the name and chemical formula of your molecule?
uppose you have two identical 1.0 l sealed containers. both containers are kept at exactly 25oc. one vessel contains only neon gas at 1.5 atm, and the other contains only xenon gas at 1.9 atm. is the value for the moles of neon less than, equal to, or greater than that of xenon? explain.
Answers
The total kinetic energy of a gas's molecules is influenced by its temperature and molar mass. However, all gases have same kinetic energy average when the temperature is the same.
What is the purpose of a xenon?In some specialized light sources, xenon is employed. It is activated by an electrical discharge, which results in a stunning blue glow. Sunbed lamps, bactericidal lamps for use in food processing, and high electrical flash bulbs for photographers are all uses for xenon lamps.
What makes xenon unique?One of the noble gases, xenon is odorless, tasteless, colorless, and chemically inert. Although not hazardous by itself, its components are potent oxidizers that are extremely toxic.
To know more about Xenon visit:
https://brainly.com/question/5516586
#SPJ4