Answers
Answer: B. 4
Explanation:
Related Questions
You need to produce a buffer solution that has a pH of 5.09. You already have a solution that contains 10. mmol m m o l (millimoles) of acetic acid. How many millimoles of acetate (the conjugate base of acetic acid) will you need to add to this solution? The pKa p K a of acetic acid is 4.74.
Answers
The number of millimoles of acetate (the conjugate base of acetic acid) needed is 3162.28 mmol
What is pH?This is simply a measure of the acidity / alkalinity of a solution.
The pH measures the hydrogen ion concentration while the pOH measures the hydroxide ion concentration
pH for buffer solutionpH = pKa + Log[conjugate]/[acid]
How to determine the number of millimoles of acetate (conjugate )From the question given above, the following data were obtained:
pH = 5.09pKa = 4.74Millimole of acid = 10 mmol Millimole of conjugate =?pH = pKa + Log[conjugate]/[acid]
5.09 = 4.74 + Log[conjugate]/ 10
Collect like terms
5.09 - 4.74 = Log[conjugate] / 10
0.35 = Log[conjugate] / 10
Cross multiply
Log[conjugate] = 0.35 × 10
Log[conjugate] = 3.5
Take the anti-log
[conjugate] = anti-log 3.5
[conjugate] = 3162.28 mmol
Thus, the number of millimoles of acetate (the conjugate base of acetic acid) needed is 3162.28 mmol
Learn more about pH of buffer:
https://brainly.com/question/21881762
#SPJ1
If one photon of radiant energy supplies 3.37 × 10−19 J, then how many photons will one mole supply
Answers
One mole of radiant energy will supply 6.02 x 10^23 photons.
How can the number of photons be determined from photon energy?
The number of photons is calculated by dividing the overall energy of a pulse by the energy of one photon included inside the pulse.
One photon: What does that mean?As a result, an electromagnetic energy particle is known as a photon. Photons have no mass, thus they move at the same rate as light.
we need to first calculate the total energy in one mole of photons,
By combining the energy per photon with Avogadro's number (6.022 x 1023), one may determine the energy of a mole of photons:
Energy per mole of photons = Energy per photon x Avogadro's number
we can calculate the energy per mole of photons:
Energy per mole of photons = 3.37 × 10−19 J x 6.022 x 10^23
Energy per mole of photons = 2.03 x 10^5 J/mol
Number of photons in one mole = Energy per mole of photons / Energy per photon
Number of photons in one mole = 2.03 x 10^5 J/mol / 3.37 × 10−19 J
Number of photons in one mole = 6.02 x 10^23 photons/mol
To know more about the photon visit:
https://brainly.com/question/12015967
#SPJ1
PLEASE HELP!!!
How many calories are in 4,180 joules?
Answers
Answer:
To convert joules to calories, you can use the conversion factor:
1 calorie = 4.184 joules
To find out how many calories are in 4,180 joules, divide the given value by the conversion factor:
4,180 joules / 4.184 joules per calorie = 0.9 calories (approximately)
Therefore, there are approximately 0.9 calories in 4,180 joules.
Formula for dinitrogen monoxide
Answers
The formula for dinitrigen monoxide is \(\fbox{N2O}\)
It is also called as nitrous oxide .
It is colorless gas
It’s structure is linear.
Answer:
the formula is N₂O
Explanation:
Do two objects with the same mass always have the same volume? Explain
Answers
Answer:
They have same volume
Explanation:
\({ \tt{volume = \frac{mass}{density} }} \\ \)
These objects will have the same volume (amount of space occupied) irrespective of their shapes. This occurs if only occupy fluids of the same density.
Why is there an imbalance in the carbon cycle?
Answers
At −12.5 ∘C, a common temperature for household freezers, what is the maximum mass of fructose (C6H12O6) you can add to 2.50 kg of pure water and still have the solution freeze? Assume that fructose is a molecular solid and does not ionize when it dissolves in water.
Answers
The maximum mass of fructose that can be added to 2.50 kg of pure water and still have the solution freeze at -12.5°C is 0 grams (or essentially 0).
To determine the maximum mass of fructose (C6H12O6) that can be added to 2.50 kg of pure water and still have the solution freeze at -12.5°C, we need to consider the concept of freezing point depression.
The freezing point depression is given by the equation:
ΔT = Kf × m
Where:
ΔT is the change in freezing point,
Kf is the cryoscopic constant for the solvent (water),
m is the molality of the solute (fructose).
Since we know the freezing point depression (ΔT) and the cryoscopic constant (Kf) for water, we can calculate the molality (m) of the fructose that will result in the desired freezing point depression.
The cryoscopic constant for water is approximately 1.86°C·kg/mol.
Given that the freezing point depression is -12.5°C, we can calculate the molality as follows:
ΔT = Kf × m
-12.5°C = (1.86°C·kg/mol) * m
Solving for m:
m = -12.5°C / (1.86°C·kg/mol)
m ≈ -6.72 mol/kg
Since molality (m) is expressed in moles of solute per kilogram of solvent, we need to convert the molality into mass of fructose.
To do this, we need to know the molar mass of fructose, which is approximately 180.16 g/mol.
Using the molality and the molar mass, we can calculate the maximum mass of fructose as follows:
Mass of fructose = m × (mass of water)
Mass of fructose = -6.72 mol/kg * 2.50 kg
Mass of fructose ≈ -16.8 mol
However, it is not physically meaningful to have a negative mass. This indicates that adding any amount of fructose will result in the solution freezing, as the fructose would further depress the freezing point below -12.5°C.
Therefore, the maximum mass of fructose that can be added to 2.50 kg of pure water and still have the solution freeze at -12.5°C is 0 grams (or essentially 0).
Learn more about fructose:
https://brainly.com/question/28117000
#SPJ1
What is the mass of 6.02 x 1024 molecules of the compound HCl?
Answers
Answer:
First, we need to determine the molar mass of HCl.
The molar mass of HCl = the mass of hydrogen (1.008 g/mol) + the mass of chlorine (35.45 g/mol) = 36.45 g/mol.
Next, we can use Avogadro's number (6.02 x 10^23 molecules/mol) to convert the number of molecules to moles:
6.02 x 10^24 molecules / 6.02 x 10^23 molecules/mol = 10 moles
Finally, we can use the molar mass to convert moles to grams:
10 moles x 36.45 g/mol = 364.5 grams
Therefore, the mass of 6.02 x 10^24 molecules of HCl is 364.5 grams.
:. It means 1 mole of Hcl
:. To find the mass of HcL
no of moles = mass/ molar mass
To get the molar mass of HCL {H=1 CL=35.5}
:. H+CL = 1+ 35.5 =36.5
So we have our molar mass and number of moles now
Then we input it in the eqn
1=x/36.5
X= 36.5g of HCl
Fe2O3 + 3 H2 –> 2 Fe + 3 H2O 6.3 g of Fe2O3 is reacted with 15.8 g of hydrogen to form iron and water. How many moles of the excess reagent are left over?
Answers
Step 1
The reaction must be written, completed, and balanced.
Fe2O3 + 3 H2 => 2 Fe + 3 H2O
The reactants: Fe2O3 and H2
------------------------
Step 2
Information provided:
The mass of Fe2O3 = 6.3 g
The mass of H2 = 15.8 g
---
Information needed:
The molar masses:
Fe2O3) 160 g/mol approx.
H2) 2.01 g/mol approx.
-----------------------
Step 3
The limiting reactant and the excess agent:
By stoichiometry,
1 mole Fe2O3 = 160 g
1 mole H2 = 2.01 g
Fe2O3 + 3 H2 => 2 Fe + 3 H2O
160 g Fe2O3 ------- 3 x 2.01 g H2
6.3 g Fe2O3 -------- X
X = 6.3 g Fe2O3 x 3 x 2.01 g H2/160 g Fe2O3 = 0.24 g
For 6.3 g Fe2O3, 0.24 g of H2 is needed, but there is 15.8 g. Therefore, the limiting reactant is Fe and the excess is the H2.
Amount of the H2 leftover:
15.8 g (provided) - 0.24 g (needed) = 15.56 g (leftover)
Mass to moles:
15.56 g x (1 mole H2/2.01 g H2) = 7.74 moles
Answer: 7.74 moles of H2 are leftover
The solubility product constant of calcium sulfate, CaSO4, is 7.10 x 10-5. Its molar
mass is 136.1 g/mol. How many grams of calcium sulfate can dissolve in 75.0 L of
pure water?
Answers
Therefore, 86.1 grams of calcium sulfate can dissolve in 75.0 L of pure water at equilibrium.
What is the calcium sulfate equilibrium constant?Only a small amount of calcium sulphate is available. The equilibrium constant, also referred to as the solubility product, is written as Ks for this kind of dissolution process. Ks = 4.9 10 6 for this reaction. K s = 4.9 10 6.
The formula for calcium sulphate's solubility product constant is:
Ksp = [Ca2+][SO42-]
The solubility product constant, Ksp, is equivalent to the product of the molar concentrations of Ca2+ and SO42- at equilibrium:
[Ca2+][SO42-] = Ksp
The following calculation can be used to determine the molar solubility of calcium sulphate:
Ksp = [Ca2+][SO42-] = x²
where x is the molar solubility of calcium sulfate.
Therefore, x = √(Ksp) = √(7.10 x 10⁻⁵) = 8.43 x 10³M
m = n × M
n = V × C
Substituting the given values, we get:
n = 75.0 L × 8.43 x 10⁻³ mol/L = 0.632 mol
m = 0.632 mol × 136.1 g/mol = 86.1 g
To know more about calcium sulfate visit:-
https://brainly.com/question/7962933
#SPJ9
Please someone help me with this

Answers
Column I and Column II represents subjects and its measuring parameters respectively
1. view of Earth from space can be represent in photograph
2. amount of rainfall in an area each month for a year can be represent in line graph
3. how the constellations change position over several hours can be represent in line graph
4. percents of the most abundant metals in Earth's crust can be represent in line graph
5. percents of the different gases in the atmosphere on Mars can be represent in circle graph
6. how far a hurricane moves each hour can be represent in line graph
7. structure of the human ear can be represent in photograph
8. daily high and low tide times for a week can be represent in line graph
9. how a sound wave travels through the air can be represent in line graph
To know more about line graph visit : https://brainly.com/question/13298277
#SPJ1
4. Calculate the number of moles in each of the following quantities:
b. 1.06 x 1023 atoms of tungsten
Answers
The number of mole in 1.06×10²³ atoms of tungsten is 0.176 mole
How do i determine the number of mole?The following data were obtained from the question:
Number of atoms = 1.06×10²³ atomsNumber of mole =?The number of mole that contains 1.06×10²³ atoms of tungsten can be obtained as illustrated below:
From Avogadro's hypothesis,
6.022×10²³ atoms = 1 mole of substance
Thus, we can say that
6.022×10²³ atoms = 1 mole of tungsten
Therefore,
1.06×10²³ atoms = (1.06×10²³ atoms × 1 mole) / 6.022×10²³ atoms
1.06×10²³ atoms = 0.176 mole of tungsten
Thus, we can conclude from the above calculation that the number of mole is 0.176 mole
Learn more about mole:
https://brainly.com/question/13314627
#SPJ1
Without differentiation of cells, complex organisms would not-
Answers
The differentiation of cells is responsible for the development of specialized tissue in living organisms.
What is meant by the differentiation of cells?
The process by which dividing cells are able to change their functional or phenotypical type is called cell differentiation. All cells have presumably been derived from the stem cells and have obtained their functions as they mature.
Cellular composition is often described as a hierarchical scheme where the stem cells are at the top of the hierarchy. According to mathematical models of cell differentiation, the proliferation, death, and differentiation of an appropriately distinguished cell population are considered.
For example, the cells that are present in a stem cell population are able to differentiate into multiple types of cells. They are able to maintain their number by self-renewal.
A progenitor cell population is derived from the stem cells and it differentiates into a restricted cell lineage. Here, a mature cell population is derived from the progenitor cells and they perform some functions and then die a natural death.
Learn more about cell differentiation here: brainly.com/question/16355203
#SPJ1
A change resulting in one or more new substances being formed is a?
A. nuclear reaction
B.physical property
C. chemical change
D.physical change
Answers
Answer:
C. Chemical change
Explanation:
A physical change is where something is changed but it doesnt affect the build up of the chemical. For example, if you broke sticks and threw them on the ground, that would be a physical change because the change is happening to the physical being of the object and not its chemical buildup. However, if you lit those sticks on fire, that would be considered a chemical change because you end up with two substances, ash and the remnants of the stick. A nuclear reaction would result in something blowing up so its not that. And a physical property is like what it looks like or how it smells. Hope I helped you!
Which of the following organisms carry out photosynthesis?
Answers
Which statement is FALSE?
1) atoms in the same period have the same number of outer electrons
2) atoms in the same group behave in a similar manner during a chemical
reaction
3) the outer electrons are the electrons in the outer energy level of an atom
4) the number of outer electrons may be used to predict how an element would
react in a chemical reaction
Answers
Answer: I think the answer is 2.
Explanation:
1. Convert 67500 mg to the unit hg
Answers
Answer:
0.675
Explanation:
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale). If this is true, what determines the difference between a slate and a gneiss rock that both are formed from shale? What role does the parent rock play in determining the type of metamorphic rock that will be formed?
Answers
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale) is a true statement.
The parent rock, in this case shale, plays a significant role in determining the type of metamorphic rock that will be formed. The minerals and structure of the parent rock provide the starting material for the metamorphic rock, and the specific conditions under which the rock undergoes metamorphism determine the final characteristics of the metamorphic rock.What determines the difference between a slate and a gneiss rock that both are formed from shale?Slate, phyllite, schist, and gneiss are all types of metamorphic rocks that can be formed from shale, which is a sedimentary rock composed of clay and other fine-grained minerals. The specific type of metamorphic rock that is formed from shale depends on the conditions under which the shale undergoes metamorphism, including the temperature, pressure, and presence of fluids.
Slate is a fine-grained metamorphic rock with a uniform, flat surface and a layered structure. It is formed when shale undergoes low-grade metamorphism, which occurs at relatively low temperatures and pressures.
Therefore, Gneiss, on the other hand, is a medium- to coarse-grained metamorphic rock with a banded or wavy texture. It is formed when shale undergoes high-grade metamorphism, which occurs at higher temperatures and pressures.
Learn more about Metamorphic Rock from
https://brainly.com/question/1176274
#SPJ1
Please help me I need the answer and it’s due almost due I can’t fail

Answers
———————————————-
37.39+62.42
= 192.25 (2dp)
Do frogs start their life cycle on land or water?
Answers
The answer is water because they lay eggs in water
What is the mass of
3.45 moles of NH4NO3
Answers
The mass of 3.45 moles of NH₄NO₃ is 276.14g.
How to calculate mass?The mass of a substance can be calculated by multiplying the number of moles in that substance by its molar mass as follows:
mass = no of moles × molar mass
Moles is the amount of substance of a system which contains exactly 6.02214076 × 10²³ elementary entities such as atoms, ions, molecules, etc.
The molar mass of NH₄NO₃ = 80.043 g/mol
mass = 3.45 mol × 80.043 g/mol
mass = 276.14g
Therefore, 276.14g is the mass contained in 3.45 moles of ammonium nitrate.
Learn more about mass at: https://brainly.com/question/20970268
#SPJ1
Which of the following is an incorrect representation for a neutral atom?
36Li
613C
3063Cu
1530P
Answers
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97. The incorrect representation for a neutral atom is 36Li
To determine the correct representation for a neutral atom, we need to consider the atomic number (Z) and mass number (A) of the element. The atomic number represents the number of protons in the nucleus, while the mass number represents the sum of protons and neutrons.
Let's analyze the given representations:
36Li:
This representation suggests that the element is lithium (Li) with a mass number of 36, which is incorrect. The correct mass number for lithium is approximately 6.94.
613C:
This representation suggests that the element is carbon (C) with a mass number of 13, which is correct. Carbon has different isotopes, and 13C represents one of its stable isotopes.
3063Cu:
This representation suggests that the element is copper (Cu) with a mass number of 63, which is correct. Copper has different isotopes, and 63Cu represents one of its stable isotopes.
1530P:
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97.
Therefore, the incorrect representation for a neutral atom is 36Li, as it does not match the known properties of lithium.
For more question on atom
https://brainly.com/question/26952570
#SPJ8
A neutron is a negatively-charged particle in the atom. true or false
Answers
Answer: true
Explanation:
An empty container with a volume of 140.0 cm3 is weighed and found to have a mass of 73.3 g. The container is filled with a liquid and reweighed. The mass of the container and the liquid is 192.1 g. What is the density of the liquid to the appropriate number of significant figures
Answers
Answer:
0.420 g/ml
Explanation:
Question
An empty container with a volume of 140.0 cm3 is weighed and found to have a mass of 73.3 g. The container is filled with a liquid and reweighed. The mass of the container and the liquid is 192.1 g. What is the density of the liquid to the appropriate number of figures
density = mass/volume
d = (192.1 -73.3)/140.0 = 58.8/140.0 =0.420 g/ml
An empty container with a volume of 140.0 cm3 is weighed and found to have a mass of 73.3 g. The container is filled with a liquid and reweighed. The mass of the container and the liquid is 192.1 g.0.420 g/ml is the density of the liquid.
What is density ?The term density is defined as the mass of a substance per unit volume. density expressed in grams per cubic centimeter. The formula of density is as follows:
Density = mass/ volume
The density of something is a measure of how heavy it is in relation to its size. When an object is denser than water, it sinks; when an object is less dense than water, it floats. Density is a property of a substance that is independent of the amount of substance.
Given:
Volume = 140.0 cm³
Density = ?
mass = 73.3 g, 192.1 g.
density = mass/volume
density = (192.1 -73.3) / 140.0
= 58.8/140.0
=0.420 g/ml
Thus, The mass of the container and the liquid is 192.1 g.0.420 g/ml is the density of the liquid.
To learn more about the density, follow the link;
https://brainly.com/question/15164682
#SPJ2
I need some help on this question please!
A chemist has 3.45 x 1022 molecules of P2O5. How many grams of P2O5 does the chemist have?
Thanks to anyone who helps!
Answers
To convert the number of molecules of P₂O₅ to grams, we need to use the Avogadro's number and the molar mass of P₂O₅ .
How many grams of P₂O₅ does the chemist have according to the question?The molar mass of P₂O₅ can be calculated as follows:
2 × molar mass of P + 5 × molar mass of O
= 2 × 30.97 g/mol + 5 × 15.99 g/mol
= 61.94 g/mol + 79.95 g/mol
= 141.89 g/mol
Therefore, the molar mass of P₂O₅ is 141.89 g/mol.
Now, we can use Avogadro's number to convert the number of molecules to moles:
3.45 × 10²² molecules / 6.022 × 10²³ molecules/mol
= 0.0573 moles
Finally, we can use the molar mass of P₂O₅ to convert moles to grams:
0.0573 moles × 141.89 g/mol = 8.14 grams
Therefore, the chemist has 8.14 grams of P₂O₅ .
To know more about Avogadro's number, visit:
https://brainly.com/question/28812626
#SPJ1
if two magnets are placed on a table, which statement describes a situation with the most attraction between the two magnets
Answers
The north pole of one magnet is near the South pole of the other magnet.
The ends of a magnet are called its poles. One end is called the north pole, the other is called the south pole. If you line up two magnets so that the south pole of one faces the north pole of the other, the magnets will pull toward each other.
Use the periodic table to select the element from the drop-down menu that has the correct relative electronegativity.
yo its been 3 minutes where my answer at
Mg>
P>
C >
Br>
Answers
The correct answer based on relative electronegativity would be:
Br > P > C > Mg
What is Electronegativity?
Electronegativity is a measure of an element's tendency to attract a bonding pair of electrons towards itself when it forms a chemical bond with another element. It is a property of elements that reflects their ability to attract and hold onto electrons in a chemical bond.
Electronegativity is a measure of an element's tendency to attract a bonding pair of electrons. In general, electronegativity increases from left to right across a period in the periodic table and decreases from top to bottom within a group. Therefore, Bromine (Br) would have the highest electronegativity among the given options, followed by Phosphorus (P), Carbon (C), and Magnesium (Mg) with the lowest electronegativity.
Learn more about Electronegativity from the given link
https://brainly.com/question/24977425
#SPJ1
What mass of water can be heated from 15.0° C to 43.1° C by the addition of 1282 J?
Answers
The mass of water that can be heated from 15.0° C to 43.1° C by the addition of 1282J is 10.9grams.
How to calculate mass?The mass of water can be calculated using the following expression;
Q = mc∆T
Where;
Q = quantity of heat absorbed or releasedm = mass of substancec = specific heat capacity∆T = change in temperatureAccording to this question, a sample of water can be heated from 15.0° C to 43.1° C by the addition of 1282J. The mass of the substance can be calculated as follows;
1282 = m × 4.184 × (43.1 - 15)
1282 = 117.57m
m = 10.9grams
Learn more about mass at: https://brainly.com/question/31584398
#SPJ1
help i have no idea what i’m doing and i’m failing this class
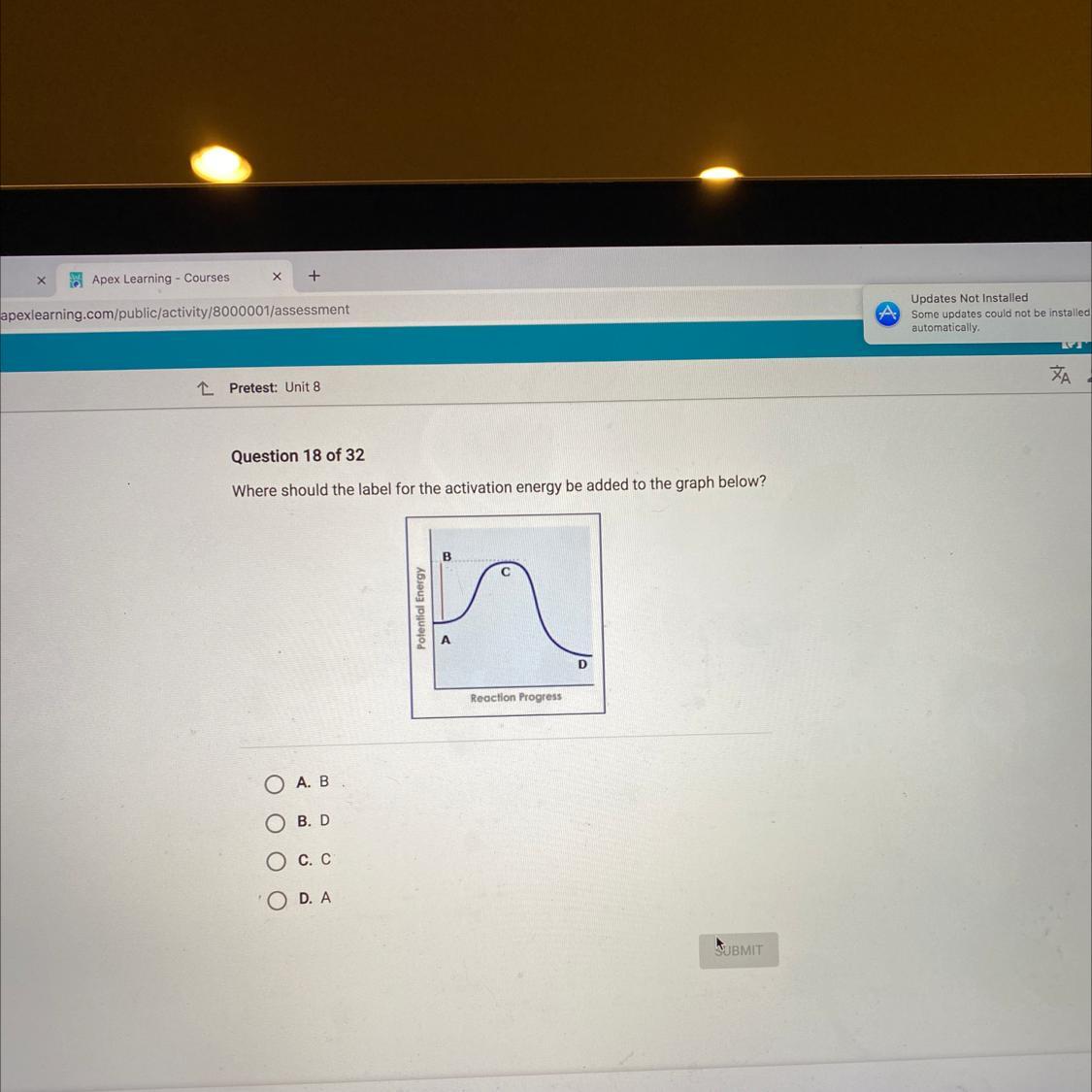
Answers
Answer:
c
Explanation:
esuba is activation energy

If chlorine had only two stable isotopes: cl-35 with a natural abundance of 70. 50% and cl-37 with a natural abundance 29. 50%. The average atomic weight of chlorine = amu.
Answers
The average atomic weight of the chlorine is 35.59amu.
How to calculate average atomic weight?Average atomic mass of a substance is the weighted average of the atomic masses of the naturally occurring isotopes of an element.
According to this question, If chlorine had only two stable isotopes: Cl-35 with a natural abundance of 70. 50% and Cl-37 with a natural abundance 29. 50%. The average atomic mass can be calculated as follows:
Percent abundance:
Cl-35 = 0.7050Cl-37 = 0.2950Average atomic mass = (0.7050 × 35) × (0.2950 × 37) = 24.675 + 10.915 = 35.59amu
Learn more about average atomic mass at: https://brainly.com/question/13753702
#SPJ1
