the decomposition of potassium chlorate(kclo3) is used as a source of oxygen in thelaboratory. how much potassium chlorate isneeded to produce 47.5 mol of oxygen?
Answers
Approximately 31.67 moles of potassium chlorate are needed to produce 47.5 mol of oxygen.
To determine how much potassium chlorate is needed to produce 47.5 mol of oxygen, we need to consider the balanced chemical equation for the decomposition of potassium chlorate:
\(2KClO_3\ - > 2KCl + 3O_2\)
From the balanced equation, we can see that 2 moles of \(KClO_3\) produce 3 moles of \(O_2\).
Therefore, we can set up the following proportion:
2 moles \(KClO_3\) / 3 moles \(O_2\) = X moles \(KClO_3\) / 47.5 moles \(O_2\)
Cross-multiplying and solving for X, we have:
\(X = (2 moles\ KClO_3 / 3 moles\ O_2) * 47.5 moles\ O_2 \\X = (2/3) * 47.5\)
X ≈ 31.67 moles \(KClO_3\)
Hence, 47.5 mol of oxygen is produced by 31.67 moles of potassium chlorate
To know more about potassium chlorate, here
brainly.com/question/15979985
#SPJ4
Related Questions
what are four states, or phrases of matter? describe the shape and volume properties of each phase, can they change or are they fixed?
Answers
Answer:
solid liquid gas and plasma
Question 5 of 10
First-quarter
moon
Solar tide
Earth
Lunar tide
Last-quarter
moon
Which term best describes the event caused by the positions of Earth, the
Moon, and the Sun shown in the diagram?
A. A solar eclipse
B. A neap tide
C. A lunar eclipse
D. A spring tide
Answers
Answer:
Solar eclipse
Explanation
Answer: it neap tide
Explanation:
suppose the sample of magnesium used in this lab was contaminated with another metal that does not react with hydrochloric acid. how would this have changed your results?
Answers
If the sample of magnesium used in a lab was contaminated with another metal that doesn't react with hydrochloric acid, then the results obtained in the experiment would be affected.
This is because the data collected during the experiment would reflect the reaction between hydrochloric acid and the contaminated sample instead of pure magnesium. As a result, the following changes in results might have been observed:
1. The mass of the contaminated sample would be higher than the mass of pure magnesium.
2. The rate of reaction between the contaminated sample and hydrochloric acid would be slower than the reaction between pure magnesium and hydrochloric acid.
3. The volume of hydrogen gas collected from the reaction would be lower than the volume of hydrogen gas collected in the reaction between pure magnesium and hydrochloric acid.
learn more about contaminated here
https://brainly.com/question/465199
#SPJ11
I'm kind of stuck with this problem.

Answers
Answer:
What are the full directions?
Answer:
Flaming
Posting on a blog
ALL CAPS
Troll
;-)
Flamewar
LOL
Explanation:
Hope this helps. Have a nice day. May it be filled with joy and happiness. Please, stay safe and wash your hands. Happy Holidays.
I NEED HELPPPPPPPPPPPPPPPPPPPPPPPPPPPPPPPPPP
Study this image.
Which statement best describes the rock shown?
1:The grains of this rock are jagged.
2:The grains in this rock are tiny.
3:This rock has a non-banded pattern.
4:The color of this rock is determined by its texture

Answers
Answer:
4
Explanation:
Answer:
c
Explanation:
Once a product is ready to be sold, engineers must explain their ideas to people who will bring the product to consumers.
Please select the best answer from the choices provided
ОТ
OF
Answers
Answer:
ot
Explanation:
there is no choices
Answer:
Advertise
Explanation:
once a product is ready to be sold, engineers explain their ideas to marketing people, who will advertise the product.
Three determinations were made of the following percentage of oxygen in mercuric oxide. The results were 7.40%, 7.43%, and 7.35%. What was the average percentage?
Answers
What would be the effect on the boiling point if you decrease the pressure over a liquid?.
Answers
Answer:
The boiling point will be reduced
Gold, which has a density of 19.32 g/cm³, is the most ductile metal and can be pressed into a thin leaf or drawn out into a long fiber. (a) If a sample of gold, with a mass of 8.489 g, is pressed into a leaf of 5.966 μm thickness, what is the area of the leaf? (b) If, instead, the gold is drawn out into a cylindrical fiber of radius 2.500 μm, what is the length of the fiber?
Answers
(a) When the density of gold is 19.32 g/\(cm^{3}\) than area of the gold leaf is approximately 0.4386 cm².
(b) The length of the gold fiber is given by h = 0.4386 cm³ / (π * (2.500 × 10⁻⁴ cm)²).
To solve these problems, we can use the formula for the volume of a shape and the given density of gold.
(a) To find the area of the leaf, we can use the formula for the volume of a rectangular shape: V = A * h, where V is the volume, A is the area, and h is the thickness.
Given the mass of gold (m = 8.489 g) and density (ρ = 19.32 g/cm³), we can find the volume: V = m / ρ.
Substituting the values, we have V = 8.489 g / 19.32 g/cm³ = 0.4386 cm³.
Since the leaf is pressed into a thin shape, we can assume it has a rectangular shape, and the volume is approximately equal to the area: A ≈ V = 0.4386 cm².
(b) To find the length of the fiber, we can use the formula for the volume of a cylindrical shape: V = π * r² * h, where V is the volume, r is the radius, and h is the length.
Given the mass of gold (m = 8.489 g) and density (ρ = 19.32 g/cm³), we can find the volume: V = m / ρ.
Substituting the values, we have V = 8.489 g / 19.32 g/cm³ = 0.4386 cm³.
The volume of a cylinder is also equal to the product of the cross-sectional area (π * r²) and the length (h), so we have: π * r² * h = 0.4386 cm³.
Substituting the radius (r = 2.500 μm = 2.500 × 10⁻⁴ cm), we can solve for the length: h = 0.4386 cm³ / (π * (2.500 × 10⁻⁴ cm)²).
To summarize:
(a) The area of the gold leaf is approximately 0.4386 cm².
(b) The length of the gold fiber is given by h = 0.4386 cm³ / (π * (2.500 × 10⁻⁴ cm)²).
To know more about density click here:
https://brainly.com/question/29775886
#SPJ11
4.
How would the electrons in a calcium atom (atomic number=12) arrange
themselves into different energy levels?
Answers
Answer:
Viewed simply, electrons are arranged in shells around an atom's nucleus. Electrons closest to the nucleus will have the lowest energy. Electrons further away from the nucleus will have higher energy. ... In a more realistic model, electrons move in atomic orbitals, or subshells.
Explanation:
Viewed simply, electrons are arranged in shells around an atom's nucleus. Electrons closest to the nucleus will have the lowest energy. Electrons further away from the nucleus will have higher energy. ... In a more realistic model, electrons move in atomic orbitals, or subshells.
What is the molarity of the solution that results when 25. 0 mL of 0. 513 M solution is diluted to 500. 0 mL? M.
Answers
Molarity is the molar concentration of the chemical species of the solute dissolved in the solvent. The molarity of the solution when diluted is 0.0257 M.
What is the relation between molarity and dilution?The molar concentration or the molarity of the solution is inversely proportional to the dilution. An increased dilution will decrease the molarity of the solution.
The molarity is given by:
\(\rm Molarity = \dfrac{\text{number of moles }}{ \text{Volume of the solution in L}}\)
Also, it can be shown as:
\(\rm M_{1}V_{1}= M_{2}V_{2}\)
Given,
\(\rm M_{1}\) = 0.513 M
\(\rm V_{1}\) = 25 mL
\(\rm M_{2}\) = ?
\(\rm V_{2}\) = 500 mL
Substitute value in the equation as:
\(\begin{aligned} \rm M_{1}V_{1}&= \rm M_{2}V_{2}\\\\0.513 \times 25 &= \rm M_{2} \times 500\\\\\rm M_{2} &= \dfrac{0.513 \times 25 }{500}\\\\&= 0.02565\;\rm M\end{aligned}\)
Therefore, 0.0257 M is the molarity after dilution.
Learn more about molarity here:
https://brainly.com/question/19004694
for mercury, the enthalpy of vaporization is 58.51 kj/mol and the entropy of vaporization is 92.92 j/k·mol. what is the normal boiling point of mercury?
Answers
K is the typical boiling point. Entropy and enthalpy of vaporization for mercury are 58.51 kJ/mol and 92.92 J/Kmol, respectively.
Which are the two vaporization methods?
Evaporation and boiling are the two types of vaporization that exist. Melting is a bulk phenomenon, whereas evaporating is a surface phenomenon. Evaporation is a phase change that takes place at a specific pressure at temperatures below boiling temperature from the liquid state to a vapor (material state below critical temperature).
How does vaporization work?
Vaporization-Affecting Factors & Examples Describe vaporization. The process by which the sol gel transforms into the vapor phase is known as vaporization. The molecular kinetic energy increases as the temperature rises.
To know more about vaporization visit:
https://brainly.com/question/14578189
#SPJ1
What are the correct coefficients when this equation is balanced?
___ Sb + __ O2 --> Sb4O6
Answers
Answer:
4 Sb, 3 \(O_{2}\)
Explanation:
On the reactant's side of the equation (the left side), there is one Antimony and one Oxygen gas molecule (\(O_{2}\)). The oxygen gas molecule is made of two atoms, so we actually have 2 oxygens on the left side. On the product's side (the right side), there are 4 antimony atoms, and 6 oxygen atoms. If we were to write it out in a certain way, it would look like this:
__Sb + __ \(O_{2}\) --> \(Sb_{4} O_{6}\)
1 Sb 4
2 O 6
To balance this equation, those numbers on either side of the elements must equal each other. We can accomplish this with the proper coefficients. If we put a 4 in front of the antimony, it means this:
4 Sb + __ \(O_{2}\) --> \(Sb_{4} O_{6}\)
4 Sb 4
2 O 6
And the antimony is now balanced.
Now we must balance the oxygen. There are 6 oxygens on the product's side but only 2 on the reactant's side. To fix this, simply multiply the oxygen by 3:
4 Sb + 3 \(O_{2}\) --> \(Sb_{4} O_{6}\)
4 Sb 4
6 O 6
3 * 2 = 6, so now oxygen is balanced, and the equation is now correct.
An ethylene glycol solution contains 30.8 g of ethylene glycol (C2H6O2) in 96.6 mL of water. (Assume a density of 1.00 g/mL for water.) Determine the freezing point of the solution. Determine the boiling point of the solution
Answers
The freezing point of the solution is -11.8 °C.
The boiling point of the solution is 103.31 °C.
To determine the freezing point of the solution, we can use the equation:
ΔTf = Kf * m
where:
ΔTf is the freezing point depression,
Kf is the cryoscopic constant (for water, Kf = 1.86 °C/m),
m is the molality of the solution (moles of solute per kilogram of solvent).
First, let's calculate the molality (m) of the solution:
Molar mass of ethylene glycol (C2H6O2):
C = 12.01 g/mol
H = 1.01 g/mol (x 6) = 6.06 g/mol
O = 16.00 g/mol (x 2) = 32.00 g/mol
Total molar mass = 12.01 g/mol + 6.06 g/mol + 32.00 g/mol = 50.07 g/mol
Number of moles of ethylene glycol (C2H6O2) = mass / molar mass
Number of moles = 30.8 g / 50.07 g/mol = 0.615 mol
Mass of water = volume x density = 96.6 mL x 1.00 g/mL = 96.6 g
Now, let's calculate the molality:
Molality (m) = moles of solute / mass of solvent (in kg)
Molality = 0.615 mol / 0.0966 kg = 6.36 mol/kg
Now we can calculate the freezing point depression (ΔTf):
ΔTf = Kf * m
ΔTf = 1.86 °C/m * 6.36 mol/kg = 11.8 °C
To find the freezing point of the solution, subtract the freezing point depression from the freezing point of pure water (0 °C):
Freezing point = 0 °C - 11.8 °C = -11.8 °C
To determine the boiling point of the solution, we can use the equation:
ΔTb = Kb * m
where:
ΔTb is the boiling point elevation,
Kb is the ebullioscopic constant (for water, Kb = 0.52 °C/m),
m is the molality of the solution (same value as calculated before: 6.36 mol/kg).
ΔTb = 0.52 °C/m * 6.36 mol/kg = 3.31 °C
To find the boiling point of the solution, add the boiling point elevation to the boiling point of pure water (100 °C):
Boiling point = 100 °C + 3.31 °C = 103.31 °C
To know more about ethylene glycol
https://brainly.com/question/32452413
#SPJ11
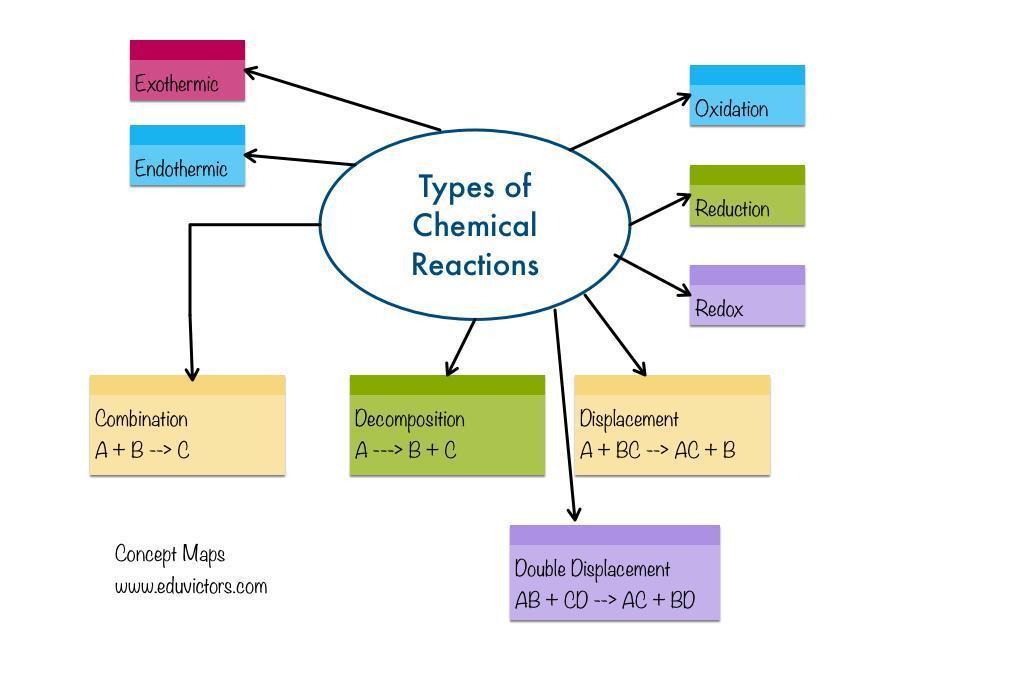
chemical change grade 10 mind map
Answers
Chemical change: A chemical reaction is the change of one chemical substance into another chemical substance. For instance: The rusting of iron, the curdling of milk, the digestion of food, breathing, etc.
What is a chemical reaction?
A chemical reaction results in a chemical change because a new material has entirely different properties from the original substance. In a chemical reaction, atoms rearrange themselves.Reactants are the chemicals that participate in a chemical reaction.Products are the new compounds created as a result of a chemical process. An illustration of a chemical reaction is burning magnesium in the air to produce magnesium oxide.2Mg(s) + O2(g) △→ 2MgO(s)The magnesium ribbon is cleaned with sandpaper before being burned in the air. This cleans the magnesium ribbon's surface of the basic magnesium carbonate protecting coating.Reactant: Materials that participate in a chemical reaction are referred to as reactants. Mg and O2, as an example.A product is a newly created substance that results from a chemical reaction. Example: MgO.A chemical reaction is the change of one chemical substance into another chemical substance.
To learn more about chemical reactions, refer to:
https://brainly.com/question/1222323
#SPJ9

Chemical change is the change chemical substance is transformed into another chemical substance.It is irreversible in nature , for example Reaction of medicine in body , milk to curd etc.
What is the difference between chemical and Physical change?1)Physical change temporary or reversible in nature but chemical change is irreversible in nature
2)In physical change there no new product is formed but in chemical change there formation of new product takes .
3) Physical change is change sin shape ,size or state for example freezing of water , melting of wax , and example of Chemical change are Burning of coal, digestion of food
to learn more about chemical change click here https://brainly.com/question/28089135
#SPJ9
what are the charecteristics of halogens
Answers
Answer: Halogens are highly reactive, and they can be harmful or lethal to biological organisms in sufficient quantities. This reactivity is due to high electronegativity and high effective nuclear charge. Halogens can gain an electron by reacting with atoms of other elements. Fluorine is one of the most reactive elements.
Brainliest pwease if i am successful in helping you!
ฅ/ᐠ。ꞈ。ᐟ\ฅ
How many moles of oxygen are in a 13g solution of nano3 solution? (molar mass of nano3 = 85g/mol)
Answers
STEP 1: To solve this question, we must first find the number of moles in a 13g solution of NaNO3 (molar mass = 85g/mol):
moles of NaNO3 = mass / molar mass
moles of NaNO3 = (13g) / (85g/mol)
STEP 2: The ratios of the compound are 3 moles of oxygen, 1 mole of sodium, and 1 mole of nitrogen. As such, for every 1 mole of NaNO3, there are 3 moles of oxygen, and the answer is:
moles of oxygen = (3) x (13/85)
What is molar mass?
Molar mass can be defined as 'mass per mole.' In other words, molar mass is the sum of the mass of all the atoms found in one mole's worth of a substance. It is expressed in units of grams per mole.
Molar mass is depicted for elements or molecules. In the case of single elements or individual atoms, the molar mass would just be the element's mass expressed in atomic mass units. In other words, the atomic mass and the molar mass of an individual atom are exactly equal.
When individual atoms are bonded to form a molecule, the molar mass represents the mass of all the atoms. Molar mass is different from molecular mass in that molecular mass is the mass of a given molecule, whereas molar mass is the mass of one mole of the given molecule.
Learn more about molar mass
https://brainly.com/question/837939
#SPJ4
a 0.08439 g sample of gas occupies 10.0 ml at 292.0 k and 1.10 atm. upon further analysis, the compound is found to be 13.068% c and 86.932% br. what is the molecular formula of the compound?
Answers
The molecular formula of the compound of molecular system is the range of atoms in one molecule of a compound best fueloline regulation relates the macroscopic homes of best gases.it describe the behaviour and homes of the fueloline C2Br2.
Ideal fueloline regulation, PV=nRTHere, p = 1.1 atmv = 10mlR=8.31m3pa/kmolt=292.0kn=0.08439gNow, the atomic weight of C =13.068/12.010=1.08802The atomic weight of Br= 86.932/79.904= 1.0879Atomic weight is the mass of 1 atom of an element.The ratio of c and Br is 1.08802/1.0879= 1/1.This is near a 1:1 ratio.Molar mass of the compound is the mass of the pattern of the compound divided through the quantity of the substance.The molar mass of CBr is 91.9147.So, the use of all of the expressions we knew that the molecular system of compound is C2Br2.Read more about compound:
https://brainly.com/question/26487468
#SPJ4
A student dissolves a spoon full of table salt (sodium chloride) in a glass of water.
After the salt is completely dissolved, the major entities in the glass (other than the
water itself) are:
(select all that apply)
Sodium chloride crystals
Aqueous sodium ions
Chlorine gas
Solid sodium metal
Liquid sodium metal
Aqueous chloride ions
Answers
Answer:
Aqueous sodium ions, Aqueous chloride ions
Explanation:
Sodium chloride dissociates into Na+ and Cl- ions in water
Sodium chloride crystals may also be present in case of saturated solution
is zinc a basic radical or acid radical?
Answers
Answer:
depends on the element it's reacting with..
Explanation:
if the another element requires less energy to loose electron then zinc will become negatively charged and hence becomes acidic radical..
normally if it is reacting with element with valency 4 and Atomic number less than it(Ti,Si,C) then it will lose electron and gain positive sign and henve becomes basic radical..
My teacher says protons and electrons are always equal to each other. So does that mean every element has a charge of 0? No, that can’t be.. but I’m still confused. Please help!
Answers
Answer: Your teacher is correct, although yes not every element has a charge of 0.
Explanation:
Atoms are electrically neutral because they contain equal quantities of positively charged protons and negatively charged electrons. Electrons and protons have equal but opposite charges, so the result is no net charge.
A 1.000-g sample of lead metal reacted with oxygen gas to give 1.154g of product. Calculate the empirical formula of the lead oxide.
Answers
To determine the empirical formula of the lead oxide, we need to find the mole ratios of lead and oxygen in the product.
First, we need to determine the moles of each element present in the product:
Mass of lead = 1.154 g - x (where x is the mass of oxygen in the product)
Mass of oxygen = x
Next, we need to convert the masses of lead and oxygen to moles:
moles of lead = (1.154 g - x) / 207.2 g/mol
moles of oxygen = x / 16.00 g/mol
We can set up a ratio of moles of lead to moles of oxygen:
(1.154 g - x) / 207.2 g/mol : x / 16.00 g/mol
Cross-multiplying and simplifying:
(1.154 g - x) x 16.00 g/mol = 207.2 g/mol x
18.464 g - 16.00 x = 207.2 x
191.736 g = 223.2 x
x = 0.859 g
So, the mass of oxygen in the product is 0.859 g. The mass of lead in the product is 1.154 g - 0.859 g = 0.295 g.
Now we can calculate the moles of each element in the product:
moles of lead = 0.295 g / 207.2 g/mol = 0.001422 mol
moles of oxygen = 0.859 g / 16.00 g/mol = 0.0537 mol
To find the empirical formula, we need to divide the moles of each element by the smallest number of moles:
0.001422 mol / 0.001422 mol = 1
0.0537 mol / 0.001422 mol = 37.7
Rounding to the nearest whole number, we get the empirical formula PbO38.
S- A simple machine which has mechanical advantange 4 and velocity ratio 5 calculate the of the efficiency simple machine.
Answers
A simple machine which has mechanical advantange 4 and velocity ratio 5, the efficiency is 80%.
The effectiveness with which a machine transforms input energy into usable output energy is known as efficiency.
It is a proportion of the machine's useful work or energy output to its overall work or energy input. A percentage is a common way to describe effectiveness.
The efficiency of a simple machine, we can apply the formula:
Efficiency = (Mechanical Advantage / Velocity Ratio) × 100%
Given that
Mechanical advantage = 4 and
Velocity ratio = 5
We can substitute these values into the formula to find the asked efficiency.
Efficiency = (4 / 5) × 100%
Efficiency = 0.8 × 100%
Efficiency = 80%
Therefore, the efficiency of the simple machine is 80%.
For more details regarding efficiency, visit:
https://brainly.com/question/31458903
#SPJ1
blood test indicates the presence of a particular disease 93% of the time when the disease is actually present. The same test indicates the presence of the disease 0.4% of the time when the disease is not present. Three percent of the population actually has the disease. Calculate the probability that a person has the disease given that the test indicates the presence of the disease. Give your answer in decimal form, rounding to four decimal places.
Answers
The probability that a person has the disease given that the test indicates the presence of the disease is approximately 0.9968
To calculate the probability that a person has the disease given that the test indicates the presence of the disease, we can use Bayes' theorem.
Let's denote:
A = Event of having the disease
B = Event of the test indicating the presence of the disease
We are given the following probabilities:
P(A) = 0.03 (3% of the population actually has the disease)
P(B|A) = 0.93 (the test indicates the presence of the disease 93% of the time when the disease is actually present)
P(B|not A) = 0.004 (the test indicates the presence of the disease 0.4% of the time when the disease is not present)
We need to find P(A|B), the probability that a person has the disease given that the test indicates the presence of the disease.
Applying Bayes' theorem:
\(P(A|B) = (P(B|A) * P(A)) / P(B)\)
To calculate P(B), we can use the law of total probability:
\(P(B) = P(B|A) * P(A) + P(B|not A) * P(not A)\)
\(P(not A) = 1 - P(A) = 1 - 0.03 = 0.97\)
Substituting the values into the equation:
\(P(B) = (0.93 * 0.03) + (0.004 * 0.97) ≈ 0.0279\)
Now, calculating P(A|B):
\(P(A|B) = (0.93 * 0.03) / 0.0279 ≈ 0.9968\)
Therefore, the probability that a person has the disease given that the test indicates the presence of the disease is approximately 0.9968 (rounded to four decimal places).
Learn more about disease from below link
https://brainly.com/question/1268202
#SPJ11
This image models…
diffraction
reflection
refraction
absorption

Answers
Answer:
reflection is correct answer by my views
As the psub(K,a) of a series of weak acids increases, the strength of their corresponding conjugate bases will
Answers
For a series of weak acids, the increase in pKa means that the conjugate bases become more stable.
What is pKa?The term pKa refers to the negative logarithm of the acid dissociation constant. This is a constant that shows the extent to which an acid is dissociated in solution.
As the pKa of a series of weak acids increases, the conjugate bases become more stable.
Learn more about pKa:https://brainly.com/question/13178964
What is the most common solvent that is used in a solution?
Answers
Answer:
It's water.
Explanation:
Water is also called as universal solvent, because it's literally almost the only one we use.
Sorry if I spelled something wrong. I'm Dominican so yeah . I hope this helps you :)
Water
Explanation :
This is because water can act either as a base or an acid based on its stable pH. It is also termed as the universal solvent.
calculate the umber of moles in o.293kg of pb as well as number of atoms {pb =207, avogaoho's constant = 6.02x10 rise to power 23}
Answers
1. The number of mole in 0.293 Kg of Pb is 1.42 mole
2. The number of atom in 0.293 Kg of Pb is 8.55×10²³ atoms
1. How to determine the number of mole
The numbe of mole in the 0.293 Kg of Pb can be obtained as follow:
Mass of Pb = 0.293 Kg = 0.293 × 1000 = 293 gMolar mass of Pb = 207 g/mol Number of mole =?Mole = mass / molar mass
Number of mole of = 293 / 207
Number of mole = 1.42 mole
Thus, the number of mole is 1.42 mole
2. How to determine the number of atoms
The number of atoms can be obtained as follow:
From Avogadro's hypothesis,
1 mole of Pb = 6.02×10²³ atoms
Therefore,
1.42 mole of Pb = (1.42 mole × 6.02×10²³ atoms) / 1 mole
1.42 mole of Pb = 8.55×10²³ atoms
Thus, the number of atoms is 8.55×10²³ atoms
Learn more about number of moles and atoms:
https://brainly.com/question/13314627
https://brainly.com/question/20712650
#SPJ1
Why do warm currents begin near the equator?
1. Ecuador recieves the most direct sunlight.
2. Scientists are not sure yet.
3. All currents, warm and cold, begin near the equator.
4. Because cold air rises and hot air descends.
Answers
Answer:
I would go with A
Explanation:
Because the earths equator is warmed by most direct rays of the sun, air a the equator is hotter than air further north or the south. The hotter air rises up at the equator and as colder air moves in to take its place, the wind begins to blow and push the ocean into waves and currents
Answer:
A
Explanation:
Because the earths equator is warmed by most direct rays of the sun which make it warm and goes up and go to cold and go down to Antarctica and come back up warm because and the most direct rays.
Zn(s) + Cu²*(aq) → Zn²+ (aq) + Cu(s)
What is the net potential (E) for the overall reaction?
(1) -0.42 V
(2)-1.10 V
(3) +1.10 V
(4) +0.42 V
Answers
The correct answer is option +1.10 V which is in option 3. The net potential (E) for the overall reaction can be determined by subtracting the reduction potential of the anode from the reduction potential of the cathode.
In this case, the reduction half-reactions and their corresponding reduction potentials are:
Zn²+(aq) + 2e- → Zn(s) E° = -0.76 V (reduction potential for Zn²+) Cu²+(aq) + 2e- → Cu(s) E° = +0.34 V (reduction potential for Cu²+)
To obtain the net potential, one has to subtract the anode reduction potential from the cathode reduction potential:
E = E°(cathode) - E°(anode) E = +0.34 V - (-0.76 V) E = +1.10 V
Therefore, the correct answer is option (3) +1.10 V.
Learn more about cell potential here
https://brainly.com/question/17168003
#SPJ1