Answers
Answer:
B Catalyst
Explanation:
catalyst is a substance that can be added to a reaction to increase the reaction rate without getting consumed in the process. Catalysts typically speed up a reaction by reducing the activation energy or changing the reaction mechanism. Enzymes are proteins that act as catalysts in biochemical reactions.That is why B is the right answer.
Related Questions
What mass of aluminum has a total nuclear charge of 1.6 CC? Aluminum has atomic number 13. Suppose the aluminum is all of the isotope with 14 neutrons. slader
Answers
Answer:
aluminium is all of isotope with 14 neutrons .
Cold-winter survival experts do not recommend eating snow for hydration. One reason for this is the energy it takes to increase temperature of the snow, melt it, and then increase the temperature of the resulting water to body temperature. Calculate the total amount of heat to convert 2.000 kg of snow at -15.00 0C to liquid water at 38.00 0C.
POTENTIALLY HELPFUL INFORMATION:
The heat of fusion for water is 1.44 kcal/mol.
The specific heat capacity of solid water is 0.488 cal/g0C.
The specific heat capacity of liquid water is 1.00 cal/g0C.
The freezing point of water is 00C (also known as melting point).
Answers
Answer:
The total amount of heat is 250.64 kcal.
Explanation:
Given that,
Total amount of heat = 2.000 kg
Temperature of snow = -15.00°C
Temperature of water = 38.00°C
Heat of fusion of water = 1.44 kcal/mol
Specific heat of solid water = 0.488 cal/g°C
Specific heat of liquid = 1.00 cal/g°C
We need to calculate the heat required to bring temperature -15°C to 0°C snow
Using formula of heat
\(Q_{1}=mc_{p}\Delta T\)
Put the value into the formula
\(Q_{1}=2000\times0.488\times(0+15)\)
\(Q_{1}=14640\ cal\)
\(Q_{1}=14.640\ kcal\)
We need to calculate the heat required to convert 0°C snow into 0°C water
Using formula of heat
\(Q_{2}=ml\)
Put the value into the formula
\(Q_{2}=\dfrac{2000}{18}\times1.44\)
\(Q_{2}=160\ kcal\)
We need to calculate heat required to increases temperature of water from 0°C to 38°C
Using formula of heat
\(Q_{3}=mc_{p}\Delta T\)
Put the value into the formula
\(Q_{3}=2000\times1.00\times(38-0)\)
\(Q_{3}=76000\ cal\)
\(Q_{3}=76\ kcal\)
We need to calculate the total heat
Using all heat amount
\(Q=Q_{1}+Q_{2}+Q_{3}\)
Put the value into the formula
\(Q=14.640+160+76\)
\(Q=250.64\ kcal\)
Hence, The total amount of heat is 250.64 kcal.
The normal boiling point of benzene is 80.1°C. What is its enthalpy of vaporization if the vapor pressure at 26.1°C is 100 torr?
Answers
The heat of vaporization of benzene is required.
The heat of vaporization of benzene is 33009 J/kg.
\(T_0\) = Normal boiling point = 80.1+273.15 K
\(T_B\) = Boiling point at given pressure = 26.1+273.15 K
\(R\) = Gas constant = 8.314 J/mol K
\(P\) = Pressure at given \(T_B\) = 100 torr
\(\Delta H\) = Heat of vaporization
From the Clausius–Clapeyron equation
\(\dfrac{1}{T_B}=\dfrac{1}{T_0}-\dfrac{R\ln(\dfrac{P}{P_0})}{\Delta H}\\\Rightarrow \Delta H=\dfrac{R\ln\dfrac{P}{P_0}}{\dfrac{1}{T_0}-\dfrac{1}{T_B}}\\\Rightarrow \Delta H=\dfrac{8.314\times \ln\left(\frac{100}{760}\right)}{\frac{1}{80.1+273.15}-\frac{1}{26.1+273.15}}\\\Rightarrow \Delta H=33008.99\ \text{J/kg}\)
The heat of vaporization of benzene is 33009 J/kg.
Learn more:
https://brainly.com/question/13878485
https://brainly.com/question/1077674
balance the chemical reaction ___ Al + ___ FeO → ___ Al2O3 + ___ Fe
Answers
Answer:
2Al+3FeO→Al2O3+3Fe
Explanation:
Basically, to get this answer, you need to balance the amount of Aluminum to the ammount on the other side which is 2 so you need 2 Al to balance the reaction correct, next you move on to the amount of Oxygen in the reaction, there are three Oxygen’s on the right and one on the left, so you need 3 FeO in order for the Oxygens to be balanced. Now that the Iron is unbalanced on both sides, you need 3 Fe (on the right) in order for the equation to be balanced.
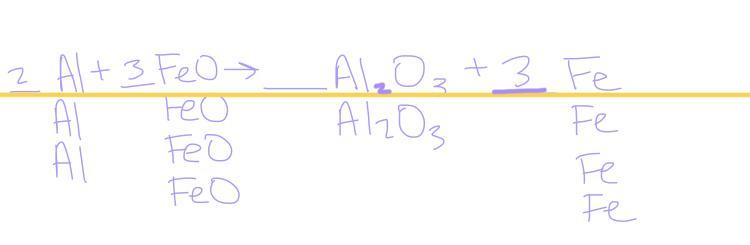
The balanced chemical reaction is \(2Al+3FeO\) → \(Al_2O_3+3Fe\)
What is a balanced chemical reaction?A balanced chemical equation occurs when the number of atoms involved on the reactants side is equal to the number of atoms on the products side.
To balance a reaction means to make the number of atoms the same on both the reactants and products sides. To do so, coefficients need to be added to the chemical equation.
Hence, the balanced the chemical reaction is \(2Al+3FeO\) → \(Al_2O_3+3Fe\).
Learn more about the balanced chemical reaction here:
https://brainly.com/question/15196081
#SPJ2
While isobaric heat can be measured by using the coffee cup calorimeter, what kind of device would be needed to measure the reaction heat under isochoric condition? Please search literature to answer the question.
To measure the reaction heat more accurately at isobaric condition, what modification(s) would you suggest making on the coffee cup calorimeter? Please justify the suggested change(s).
Answers
To measure reaction heat under isochoric conditions, a bomb calorimeter is needed.
This device is designed to maintain a constant volume (isochoric) during the reaction, allowing for accurate measurement of reaction heat. To improve the accuracy of the coffee cup calorimeter for measuring reaction heat under isobaric conditions, a modification that could be made is to use a stirring device to ensure uniform mixing of the reactants and to minimize heat loss to the surroundings.
Additionally, a lid with a small hole could be placed over the top of the calorimeter to prevent heat loss while still allowing for pressure equalization. These modifications would help to minimize errors in heat measurement and improve the accuracy of the results obtained.
To know more about the Calorimeter, here
https://brainly.com/question/24150308
#SPJ1
Josh heated a certain amount of blue copper sulfate crystals to get 2.1 g of white copper sulfate powder and 1.4 g of water. What is most likely the mass of the blue copper sulfate that he heated and why?
Answers
Answer: The mass of blue copper sulfate is 3.5 g
Explanation:
Law of conservation of mass states that mass can neither be created nor be destroyed but it can only be transformed from one form to another form.
This also means that total mass on the reactant side must be equal to the total mass on the product side.
The chemical equation for the heating of copper sulfate crystals is:
Let the mass of blue copper sulfate be 'x' grams
We are given:
Mass of copper sulfate powder = 2.1 grams
Mass of water = 1.4 grams
Total mass on reactant side = x
Total mass on product side = (2.1 + 1.4) g
So, by applying law of conservation of mass, we get:
Hence, the mass of blue copper sulfate is 3.5 grams
When 3.00 moles of hydrogen molecules and 1.50 moles of oxygen molecules react, they form 3.00 moles of water
according to the reaction below.
2H, + O2 + 2H2O
How many grams of oxygen were required?
Answers
Answer:
Mass = 48 g
Explanation:
Given data:
Number of moles of H₂ react= 3.00 mol
Number of moles of O₂ react= 1.50 mol
Number of moles of water formed = 3.00 mol
Mass of oxygen react = ?
Solution:
Chemical equation:
2H₂ + O₂ → 2H₂O
It is stated in given question when 1.50 moles of oxygen react 3.00 moles of water are formed. Thus, mass of 1.50 moles of oxygen is
Mass of oxygen:
Mass = number of moles × molar mass
Mass = 1.50 mol × 32 g/mol
Mass = 48 g
Which sample uses the substance(s) that Jacob and Natalie
should use to make a cold pack that will do the BEST job of
keeping food cool

Answers
The sample that uses the substance that Jacob and Natalie should use to make a cold pack that will do the best job of keeping food cool is sample 2, because it absorbs the most energy (option B).
What is endothermic process?Endothermic refers to a chemical reaction that absorbs heat energy from its surroundings. This ensures that the temperature of the surroundings is cool or has a lower temperature.
According to this question, Jacob and Natalie are asked by their science teacher to design a warming or cooling device. They make use of certain substances, however, sample 2 has the lowest final temperature of -4°C.
This shows that sample 2 absorbs the most energy, hence, would be the best for keeping the food cool.
The incomplete question is as follows:
Jacob and Natalie are asked by their science teacher to design a warming or cooling device. They decide to design a cold pack that can be used to help keep food cool. Jacob and Natalie read about different substances that can be used inside cold packs and learn that most cold packs use endothermic reactions to cool objects.
Learn more about endothermic at: https://brainly.com/question/28909381
#SPJ1
What information does a molecular formula give?
O A. The actual number of atoms in a molecule
OB. The percent composition of atoms in a molecule
O C. The lowest ratio of atoms in a molecule
O D. The order in which atoms are bonded in a molecule
Answers
Answer:
A. The actual number of atoms in a molecule
Explanation:
Molecular formula is simply defined as a type of chemical formula that describes the type and number of atoms present in a single molecule of a compound.
Looking at the options, the correct answer is option A.
Answer:
a
Explanation:
51 neutrons 38 protons 36 electrons
Answers
Answer:
copper has two naturally occurring isotopes. Cu-63 has an atomic mass of 62.9296 amu and an abundance of 69.17%. if thats what ur looking for?
Explanation:
nononononononononononon
Answers
Answer:
Explanation:
yesyesyesyesyesyesyesyesyes
Ions can conduct electricity during electrolysis because they are free to move and they are … what?
Answers
When these ions are created, they enter the solution and have the ability to conduct charge by travelling anticlockwise to the circuit's electrons.
When ions were free to move, what does that mean?Because their ions were free to move around, ionic substances conduct electricity if they are molten (liquid) or even in aqueous (dissolved in water). Ions in ionic compounds are maintained in fixed locations and are unable to move, therefore they cannot conduct electricity while solid.
How do ions that are in motion conduct electricity?Ions are all charged. As a result, anions travel in the direction of positively charged electrode whereas ions with positive charges move in the opposite direction. The charge moves together with the motion of the ions. Current is created by this charge movement
to know more about ions visit:
https://brainly.in/question/15760168
#SPJ1
in order to produce an electric current in a coil of wire , a magnet field through the coil must be
Answers
Answer:
moving
Explanation:
In order to produce an electric current in a coil of wire, a magnet field through the coil must be moving.
What is electromagnetic induction?Electromagnetic induction is a process in which electric field is produced due to the change in the value of magnetic field.
So, for the production of an electric current in a coil of wire, a magnet field should be moving through the coil of wire. Process of this electromegnetism get clear through the below attached image.
Hence magnetic field should be moving.
To kow more about electromagnetic induction, visit the below link:
https://brainly.com/question/1305957
#SPJ2
What was one idea Dalton taught about atoms?
Answers
Explanation:
All atoms of one type were identical in mass and properties.
What happens to the amount of solution when we add food colour to it?
Answers
Answer:
We need more? What else is in the question? This is unanswerable.
Explanation:
Which of the following compounds is least soluble in water?
A) iron (III) chloride
B) ammonium acetate
C) sodium hydroxide
D) magnesium carbonate
Answers
Answer:
B
Explanation:
Which statements best describe half lives of radioactive isotopes
Answers
Answer:
The half-life varies depending on the isotope.
Half-lives range from fractions of a second to billions of years.
The half-life of a particular isotope is constant.
Explanation:
Make sure you add the options
A type of emergency apparatus that can be used where oxygen may be limited or where the air might be poisoned is based on the following reaction in which CO2 produced by your own respiration reacts and O2 gas is produced. Calculate the mass of KO2 needed to produce 25 g of oxygen gas.
4 KO2(s) + 2 CO2(g) 2 K2CO3(s) + 3 O2 (g)
Answers
Answer:
so I don't know how to answer that
Which one of the following salts is least soluble in water?
1. Na2SO4
2.CaBr2
3. LiCl
4. RbI
5. PbSO4
Answers
3 attempts left Be sure to answer all parts. Which indicators that would be suitable for each of the following titrations: (a) CH3NH2 with HBr thymol blue bromophenol blue methyl orange methyl red chlorophenol blue bromothymol blue cresol red phenolphthalein (b) HNO3 with NaOH thymol blue bromophenol blue methyl orange methyl red chlorophenol blue bromothymol blue cresol red phenolphthalein (c) HNO2 with KOH thymol blue bromophenol blue methyl orange methyl red chlorophenol blue bromothymol blue cresol red phenolphthalein
Answers
An indicator usually signals the endpoint of a neutralization reaction by undergoing a color change. They aid in discovering the point of equivalence of a titration.
The kind of indicator used depends on the nature of acid/base reacted.
In the case of CH3NH2 with HBr which strong acid and weak base titration, suitable indicators include; bromophenol blue, methyl orange, methyl red, and chlorophenol blue.
In the case of HNO3 with NaOH, this is a strong acid, strong base titration hence phenolphthalein, methyl red, chlorophenol, and bromothymol blue cresol red blue are suitable indicators.
In the case of HNO2 with KOH, this a weak acid, strong base titration and the suitable indicators are cresol red and phenolphthalein.
For more information on titration see
https://brainly.com/question/22536636
What summarizes the process of cellular respiration in plants and animals
Answers
In both plants and animals, the process of cellular respiration may be broken down into the following steps: Oxygen + carbon dioxide.
When plant cells undergo cellular respiration, oxygen is taken in through the plant's leaves from the surrounding atmosphere, and carbon dioxide is exhaled back into the atmosphere as a byproduct of the process. In contrast, when animal cells undergo cellular respiration, oxygen is taken in through the lungs, and carbon dioxide is exhaled through the lungs.
Therefore, a quick review, throughout the process of cellular respiration, plants and animals trade oxygen and carbon dioxide with one another.
Therefore, we may draw the following conclusion: Oxygen + carbon dioxide is a shorthand for the process of cellular respiration in plants and animals.
Know more about cellular respiration at,
https://brainly.com/question/13721588
During the electrolysis of molten sodium chloride, which ion discharges at the anode?
Answers
Answer:
it should be chlorine gas
Explanation:
What volume will 3.0 moles of O2 gas occupy at S.T.P?
Answers
Answer:
3 moles of oxygen at STP will occupy a volume of 3×22. 4=67. 2 L.
Explanation:
carbon disulfide burns in oxygen to yield carbon dioxide and sulfur dioxide according to the fallowing chemical equation Cs2+3O2=CO2+2SO2
Answers
A. The limiting reactant would be oxygen.
B. The mole of excess reactant (\(CS_2\)) would be 0.67 mol.
C. 0.33 mol \(CO_2\) and 0.67 mol \(SO_3\) would be formed.
Stoichiometric problemCarbon disulfide burns in oxygen to produce carbon dioxide and sulfur dioxide according to the balanced equation below:
\(CS_2(l) + 3O_2(g) -- > CO_2(g) + 2SO_2(g)\)
The mole ratio of the carbon disulfide and the oxygen gas is 1:3. In other words, every mole of \(CS_2\) requires 3 moles of \(O_2\) for complete combustion.
A. From the established mole ratio, if 1.00 mol of \(CS_2\) reacts with 1.00 mol of \(O_2\), the limiting reactant would be \(O_2\) because 3 moles of it are required for every mole of \(CS_2\).
B. The excess reactant is, thus, \(CS_2\). 1.00 mol of \(O_2\) would require:
3 mol \(O_2\) = 1 mol \(CS_2\)
1 mol \(O_2\) = 1 x 1/3
= 0.33 mol of \(CS_2\)
But 1 mole of \(CS_2\) reacted. The excess mole of \(CS_2\) would be: 1 - 0.33 = 0.67 mol.
C. From the equation of the reaction, 3 mole of oxygen produces 1 mole of \(CO_2\). Thus, 1 mole of oxygen would produce:
3 mol \(O_2\) = 1 mol \(CO_2\)
1 mol \(O_2\) = 1 x 1/3
= 0.33 mol \(CO_2\)
Also, 3 mol oxygen produces 2 moles \(SO_2\). Then 1 mol oxygen will produce:
2 x 1/3 = 0.67 mol
Thus, 0.33 mol \(CO_2\) and 0.67 mol \(SO_3\) will be formed from 1 mol of \(O_2\).
More on stoichiometric problems can be found here: https://brainly.com/question/14187102
#SPJ1
Carbon disulfide burns in oxygen to yield carbon dioxide and sulfur dioxide according to the following chemical equation. CS2(l) + 3O2(g) → CO2(g) + 2SO2(g)
a. If 1.00 mol CS2 reacts with 1.00 mol O2, identify the limiting reactant.
b. How many moles of excess reactant remain?
c. How many moles of each product are formed?
Hotter things have more energy than colder things. this is science middle school
Answers
Answer:
true
Explanation:
Hotter things have more heat energy than colder things. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). ... The more heat you supply, the faster the molecules move and the further apart they get.
Answer: Depends on the situation.
Explanation: Hotter things do have more energy than colder things. But, if the mass of the colder thing is bigger, it really depends. If the colder thing have way more particles than the hotter thing, the colder thing may have more energy.
Help!!! A radio station transmits its signal at 93.1 MHz. What is the wavelength signal ?

Answers
Answer:
3.22m
Explanation:
c=3×10^8m/s=speed of the light
lambda= wavelength=?
frequency=?
lambda=c÷frequency
lambda=(3×10^8)÷(93.1×10^6)
lambda=3.22m
What is the first step for response to an emergency situation?
Answers
Answer:
Back away from the situation and tell the supervisor/teacher
which is an example of a colloid? a mixture that settles out, a mixture that scatters light, a mixture that is separated by filtration, or a salt and water mixture?
Answers
These substances have dispersed particles that are large enough to scatter light, making the beam visible. Therefore, out of the options provided, a mixture that scatters light is an example of a colloid. Option B)
A colloid is a type of mixture in which particles are dispersed throughout a medium, creating a homogeneous appearance. Unlike solutions, where the particles are completely dissolved, and suspensions, where the particles settle out, colloids have particles that are larger than those in solutions but smaller than those in suspensions. One characteristic of colloids is that they can scatter light due to the size of the particles. This scattering of light is known as the Tyndall effect. Examples of colloids include milk, fog, and aerosol sprays. These substances have dispersed particles that are large enough to scatter light, making the beam visible. Therefore, out of the options provided, a mixture that scatters light is an example of a colloid. Therefore option B) is correct
For more question on mixture
https://brainly.com/question/24647756
#SPJ8
Note Complete Question
which is an example of a colloid?
a mixture that settles out,
b mixture that scatters light,
c mixture that is separated by filtration,
d salt and water mixture?
Balance the equation
Ca(s) + H3PO4(aq) ---->Ca3(PO4)2(s) + H2(g)
Answers
A number should be rounded up if________? it is the first digit, the number after it is between 0 and 4, the number after is between 5 and 9, it is the last digit
Answers
Answer:
a number should be rounded up it is 5 or higher