Answers
The balanced molecular equation for the reaction between solid sodium bicarbonate and hydrochloric acid to produce carbon dioxide gas, water and aqueous solution of sodium chloride is
NaHCO₃(s) + HCl(aq) —> CO₂(g) + H₂O(l) + NaCl(aq)
The balanced equation can be written as follow:
Sodium bicarbonate => NaHCO₃
Hydrochloric acid => HCl
Carbon dioxide => CO₂
Water => H₂O
Sodium chloride => NaCl
Sodium bicarbonate + Hydrochloric acid —> Carbon dioxide + Water + Sodium chloride
NaHCO₃(s) + HCl(aq) —> CO₂(g) + H₂O(l) + NaCl(aq)The equation above is balanced since the number of the different atoms of the elements present on both sides are equal.
Learn more: https://brainly.com/question/2399130
Related Questions
For the reaction 2H₂(g) + O₂(g) → 2H₂O(g), what volume of water vapor can be made from 100 grams of oxygen gas and an excess of hydrogen at STP? Please show work.
Answers
Answer:
140 L
Explanation:
Step 1: Write the balanced equation
2 H₂(g) + O₂(g) → 2 H₂O(g)
Step 2: Calculate the moles corresponding to 100 g of oxygen
The molar mass of oxygen is 32.00 g/mol.
\(100g \times \frac{1mol}{32.00g} =3.13mol\)
Step 3: Calculate the moles of water vapor formed
The molar ratio of oxygen to water vapor is 1:2.
\(3.13molO_2 \times \frac{2molH_2O}{1molO_2} =6.26molH_2O\)
Step 4: Calculate the volume corresponding to 6.26 moles of water vapor
1 mole of any ideal gas under STP has a volume of 22.4 L.
\(6.26mol \times \frac{22.4L}{mol} =140 L\)
I will give 20 points for correct answer. No links!
What prevents electrons from colliding with the nucleus? a) momentum b) gravity c) heat

Answers
What I know so far is that heat makes it harder for the electrons to reach the source, thus making it hard to collide with the nucleus, but I’m not sure so you can see if it’s correct.
.
Complete the equation for cellular respiration:
________ + sugar → ________ + water
oxygen, carbon dioxide
carbon dioxide, oxygen
ATP, light energy
chlorophyll, mitochondria
Answers
The equation for cellular respiration is in the given choices is option A. oxygen + sugar → carbon dioxide + water
Cellular respiratory is a chain of chemical reactions that smash down glucose to produce ATP, which may be used as power to energy many reactions throughout the body. There are 3 predominant steps of mobile respiratory: glycolysis, the citric acid cycle, and oxidative phosphorylation.
Cellular respiration is the process by the way which organic fuels are oxidized in the presence of an inorganic electron acceptor along with oxygen to provide massive quantities of power, to power the bulk manufacturing of ATP.
Learn more about cellular respiration here:-https://brainly.com/question/12311315
#SPJ1
Plants need sunlight to make food during the process of:
Photosynthesis
Respiration
Nuclear fusion
Digestion
Answers
the answer is photosynthesis
Mass of Cups & Water 142,99 g Mass of Cups 6.76 g. what is the mass of just water?
Answers
Answer:
The mass of water is 14292.24 g
Explanation:
Given data:
Mass of cups and water = 142,99 g
Mass of cups = 6.76 g
Mass of water = ?
Solution:
Mass of cups + water = mass of cups + mass of water
Now we will rearrange the formula
mass of water = (Mass of cups + water) - mass of cups
Now we will put the values.
mass of water = 142,99 g + 6.76 g
mass of water = 14292.24 g
Thus mass of water is 14292.24 g.
If the mass of 3.01 x1023 molecules of a pure substance is 8.0 g, which of the following could be the identity of the substance?
Answers
Answer:
8.0g
Explanation:
the peoples place is it okay if you are sick of the way you welcomeeee is otay Mesa or something that would make it there for a long day to have the most beautiful and 2nd century old town n or the time of year and I don't think it's a big deal to get in on my own or not at McDonald's but you have a great deal on it is a bit too much for me and my husband is 6AM a good guy and he has a great time I'm just so happy to have him on my way to work and to get a job and get it done and then we will have a better for a better life than I loveeee to be with my wife in a relationship and then she will and I will always have a relationship
CAN BOTS STOP POSTING THEIR ANSWERS; please answer my questions i need help with those two problems

Answers
Answer:
Answer is in a photo. I can only upload it to a file hosting service. link below!
Explanation:
What are the ingredients in a margaritaWhat are the ingredients in a margarita drink
Answers
Answer:
cointreau, tequila,lime juice
Drag the tiles to the correct locations on the equation. Not all tiles will be used.
Two atoms interact with each other and change as shown by the equation. Complete the equation by filling in the missing parts.
5
2
4
3
1
H+H -
H
He
Li
+
Answers
The equation in the question is: H+H → H + H Complete the equation by filling in the missing parts. missing part is 1 → H+H-2 → →3 → He.
The atomic number of hydrogen is 1, which means it has only one proton in the nucleus and one electron in its shell. Two hydrogen atoms react with each other to form helium. Helium has 2 protons and 2 neutrons in its nucleus and two electrons in its shell. Therefore, the equation is:
H + H → HeIt can be seen that:1. H + H (Reactants)
2. → (Yields or Reacts to form)
3. He (Product)Therefore, the tiles will be arranged as shown below: 1 → H+H-2 → →3 → He
For more question atomic number
https://brainly.com/question/16858932
#SPJ8
cell and chromosome characteristics?
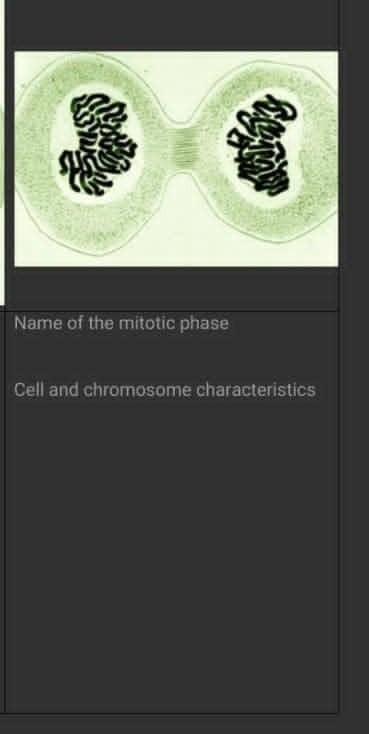
Answers
Answer:
hope the inserted image will help :)
Explanation:


Answer: Both are centered inside the nucleus.
Explanation: The characteristics between cells and chromosomes are that they both are centered inside the nucleus (which is a cell). This characteristic applies to both plant and animal cells.
Given: H2 + O 2 → H2O1
the reaction occurs at ST.P a) Balance the chemical equation. (1 pts) b) Calculate the number of moles of the reactants needed to obtain 45 liner of H2O (2 pt) 4) Deduce the volume of the reactants (2 pts)
Answers
a) The balanced chemical equation for the reaction is: 2H₂ + O₂ → 2H₂O
b) the number of moles of O₂ required is approximately 1.004 moles.
c) approximately 45 liters of H₂ and 22.5 liters of O₂ are needed to obtain 45 liters of H₂O.
a) Balancing the chemical equation:
The balanced chemical equation for the reaction is: 2H₂ + O₂ → 2H₂O
b) Calculating the number of moles of the reactants needed to obtain 45 liters of H₂O:
From the balanced equation, we can see that for every 2 moles of H₂O produced, we need 2 moles of H₂ and 1 mole of O₂. Since the stoichiometry is based on moles, we need to convert the given volume of H2O into moles.
To convert volume to moles, we need to use the ideal gas law, PV = nRT. At standard temperature and pressure (STP), the molar volume of an ideal gas is 22.4 liters.
Given that we have 45 liters of H2O, we can calculate the number of moles as follows:
moles of H₂O = (volume of H₂O) / (molar volume at STP)
= 45 liters / 22.4 liters/mol
≈ 2.008 moles of H₂O
Since the stoichiometry of the reaction is 2 moles of H₂O for every 2 moles of H₂, we need an equal number of moles of H₂. Therefore, the number of moles of H₂ required is also approximately 2.008 moles.
For O₂, since the stoichiometry is 1 mole of O₂ for every 2 moles of H₂O, we need half the number of moles of H₂O. Thus, the number of moles of O₂required is approximately 1.004 moles.
c) the volume of the reactants:
Since the stoichiometry of the balanced equation is 2 moles of H₂for every 1 mole of O₂ and 2 moles of H₂O, we can deduce the volume of the reactants based on their molar volumes at STP.
For 2.008 moles of H₂, the volume can be calculated as follows:
volume of H₂= (moles of H₂) * (molar volume at STP)
= 2.008 moles * 22.4 liters/mol
≈ 45 liters of H₂
For 1.004 moles of O₂, the volume can be calculated similarly:
volume of O₂= (moles of O₂) * (molar volume at STP)
= 1.004 moles * 22.4 liters/mol
≈ 22.5 liters of O₂
Therefore, approximately 45 liters of H₂and 22.5 liters of O₂ are needed to obtain 45 liters of H₂O
for more questions on chemical
https://brainly.com/question/29886197
#SPJ8
This time, include both the coefficient and exponent.
Express 0.00212 in scientific notation.
[?] ×10 [?]
![This time, include both the coefficient and exponent. Express 0.00212 in scientific notation. [?] 10](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/4xBfA5Z8WDeWElviTioi7szbTVlil4E1.jpeg)
Answers
The scientific notation of 0.00212 is 2.12 × 10⁻³.
Scientific notation is a way of expressing numbers that might be too big or too small to be quite simply written in decimal shape. it is able to be known as clinical shape or popular index shape, or widespread shape within the united kingdom.
Scientific notation is a way of writing very large or very small numbers. various is written in medical notation whilst various among 1 and 10 is increased by means of electricity of 10. as an instance, 650,000,000 can be written in clinical notation as 6. five ✕ 10^eight.
The number one purpose for changing numbers into medical notation is to make calculations with unusually massive or small numbers less cumbersome. due to the fact zeros are now not used to set the decimal point, all of the digits in a variety of scientific notation are giant.
Calculation:-
Scientific notation of 0.00212 = 2.12 × 10⁻³
Learn more about scientific notation here:-https://brainly.com/question/28550656
#SPJ1
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
"Dark Gray had the most consistent temperature at 88°F" Which BEST explains why this a weak evidence statement? "Dark Gray had the most consistent temperature at 88°F" Which BEST explains why this a weak evidence statement? The evidence statement is based on bad/incorrect data. The evidence statement is untrue. The evidence statement is extra information that doesn't help answer the question. The evidence statement contains reasoning. The evidence statement contains a claim.
Answers
Answer:
the evidence statement contains reasoning.
Explanation:
the reason that this piece of information is reasoning rather than evidence is because it doesn't tell us the temperatures of the other substances (if any.) if we look at this substance and it has the most constant temperature compared to the others, how will we know if it is the most constant if we don't know the other substances' data?
this is reasoning because the evidence should tell us more about the substance and the others.
QUESTION 1
Consider the following reaction: CH4 + 202 --> 2H2O + CO2
How many moles of water can be formed from 1.1 moles of CH4?
Answers
Explanation: This one is a lot easier than it seems! All you have to do is multiply 1.1 by the mole ratio(look at the coefficients)

how many grams were dissolved to make 2.0 L of a 0.4 M KNO3 solution
Answers
Answer:80.8g
Explanation:
= . / ()

Help please! I'll give brainliest as well if you show work/explain :)

Answers
Answer:
see below
Explanation:
A. The given reaction releases energy, indicating that it is an exothermic reaction.
B. The △H value for the reaction can be written as △H = -571.7 kJ, with a negative sign indicating the energy is released.
C. The balanced equation for the reaction between hydrogen and oxygen to form water is:
2H2(g) + O2(g) → 2H2O(l)
D. To calculate the amount of energy released when 10.0g of hydrogen is reacted with an excess of oxygen, we need to first determine the amount of hydrogen involved in the reaction.
The molar mass of hydrogen is 1.008 g/mol, so 10.0 g of hydrogen is equivalent to 10.0 g / 1.008 g/mol = 9.92 mol of hydrogen.
From the balanced equation, we can see that 2 mol of hydrogen is required for every 1 mol of oxygen, so the amount of oxygen involved in the reaction is twice the amount of hydrogen.
Therefore, the amount of oxygen needed for 9.92 mol of hydrogen is 2 × 9.92 mol = 19.84 mol.
Assuming that there is an excess of oxygen, all of the hydrogen will react, so the limiting reactant is oxygen.
Using the △H value of -571.7 kJ, we can calculate the amount of energy released as follows:
-571.7 kJ / 2 mol H2 = -285.9 kJ/mol H2
So the energy released when 10.0 g of hydrogen reacts with an excess of oxygen is:
-285.9 kJ/mol H2 × 9.92 mol H2 = -2836.53 kJ
Therefore, the reaction releases 2836.53 kJ of energy when 10.0 g of hydrogen reacts with an excess of oxygen.
In addition to pH meter, what other methods and/or experimental devices may be used to determine the Ksp values of sparingly soluble electrolytes? Please give at least three examples.
What factor(s) may change Ksp values? Please elaborate your answer.
Answers
According to the solubility of of sparingly soluble electrolytes by conductometer and potentiometer.
Solubility is defined as the ability of a substance which is basically solute to form a solution with another substance. There is an extent to which a substance is soluble in a particular solvent. This is generally measured as the concentration of a solute present in a saturated solution.
The solubility mainly depends on the composition of solute and solvent ,its pH and presence of other dissolved substance. It is also dependent on temperature and pressure which is maintained.It can also be determined for electrolytes.
Learn more about solubility,here:
https://brainly.com/question/31493083
#SPJ1
Water is exposed to electromagnetic radiation of wavelength 3.80 x 10-8 m. Assume that all the radiation is absorbed and converted to heat. How many photons are required to raise the temperature of 4.48 g of water by 4.65 K?
Answers
The number of photons is obtained from the calculation as 1.68 * 10^19 photons.
What is energy?
By definition, we know that energy is the ability to do work. Now we know that we have the wavelength of the radiation can be seen from the question and the values that are given to be 3.80 x 10-8 m.
Then energy = hc/λ
h = Plank's constant
c = speed of light
λ = wavelength
E = 6.6 * 10^-34 * 3 * 10^8/ 3.80 x 10^-8
E = 5.2 * 10^-18 J
Energy of the water = mcdT
= 4.48 * 4.2 * 4.65 = 87.5 J
Number of photons required = 87.5 J/5.2 * 10^-18 J
= 1.68 * 10^19 photons
Learn more about photons:https://brainly.com/question/20912241
#SPJ1
Calculate the energy of an electron in the n = 2 level of a hydrogen atom.
Answers
Answer: The energy of an electron in the n = 2 level of a hydrogen atom is 3.40 eV.
Explanation:
Given: n = 2
The relation between energy and \(n^{th}\) orbit of an atom is as follows.
\(E = - \frac{13.6}{n^{2}} eV\)
Substitute the values into above formula as follows.
\(E = - \frac{13.6}{n^{2}} eV\\= - \frac{13.6}{(2)^{2}}\\= - 3.40 eV\)
The negative sign indicates that energy is being released.
Thus, we can conclude that the energy of an electron in the n = 2 level of a hydrogen atom is 3.40 eV.
Draw the major organic product formed in the reaction. (The reaction stoichiometry is 1 mol reactant: 1 mol Br2 .)
Answers
Answer:
Explanation:
The treatment of aldehyde or ketone in the presence of acetic acid with one mole of bromine results in ∝-halo aldehyde or ketone. However, the bromination of ketone undergoes acidic conditions. Hence, the reaction of the ketone with Bromine resulting in the Major Product is shown in the image attached below.

2.62 Predict the chemical formulas of the compounds formed by the following pairs of ions: (a) Cr3+ and Br, (b) Fe3+ and O2, (c) Hg22+ and CO2, (d) Ca2+ and CIO3, (e) NH4+ and PO³
Answers
Answer:
(a) Cr3+ and Br- will form CrBr3 (chromium(III) bromide)
(b) Fe3+ and O2- will form Fe2O3 (iron(III) oxide)
(c) Hg22+ and CO32- will form Hg2CO3 (mercury(I) carbonate)
(d) Ca2+ and ClO3- will form Ca(ClO3)2 (calcium chlorate)
(e) NH4+ and PO43- will form (NH4)3PO4 (ammonium phosphate)
Explanation:
chatGPT
The chemical formulas for the compounds formed by the given pairs of ions are: (a) CrBr3, (b) Fe2O3, (c) Hg2(CO3)2, (d) Ca(ClO3)2, and (e) (NH4)3PO4.
Explanation:(a) Cr3+ and Br- : In order to form a neutral compound, the charges of the ions must balance. The charge of Cr3+ is 3+ and the charge of Br- is 1-. To balance the charges, we need three Br- ions for every Cr3+ ion. Therefore, the chemical formula is CrBr3.
(b) Fe3+ and O2- : The charge of Fe3+ is 3+ and the charge of O2- is 2-. To balance the charges, we need two O2- ions for every Fe3+ ion. Therefore, the chemical formula is Fe2O3.
(c) Hg22+ and CO2- : The charge of Hg22+ is 2+ and the charge of CO2- is 2-. The charges are already balanced, so no extra ions are needed. Therefore, the chemical formula is Hg2(CO3)2.
(d) Ca2+ and ClO3- : The charge of Ca2+ is 2+ and the charge of ClO3- is 1-. To balance the charges, we need two ClO3- ions for every Ca2+ ion. Therefore, the chemical formula is Ca(ClO3)2.
(e) NH4+ and PO3- : The charge of NH4+ is 1+ and the charge of PO3- is 3-. To balance the charges, we need three NH4+ ions for every PO3- ion. Therefore, the chemical formula is (NH4)3PO4.
Learn more about Chemical Formulas here:https://brainly.com/question/36379566
#SPJ3
Balancing Equations 1. .
_NH4NO3 → _N20 + _H20

Answers
Answer:
_ NH4NO3 -> _ N2O + 2 H2O
Explanation:
By having 2 water molecules, we reach the 4 H atoms and 3 O atoms that are present on the left side. We don't add any other coefficients because we already have enough N atoms and we don't have to manipulate the left side at all.
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
Which statement below best describes a volatile liquid?
Question 17 options:
1.A liquid that doesn't evaporate under atmospheric pressure
2.A liquid that evaporates slowly at low temperatures
3.A liquid that evaporates rapidly at low temperatures
4.A liquid that requires a large amount of energy to evaporate
Answers
Answer:
A liquid that evaporates rapidly at low temperatures
Explanation:
Answer:
A liquid that evaporates rapidly at low temperatures.
Explanation:
A volatile liquid such as gasoline evaporates easily. Its chemicals aren't held together as strongly, and room temperature is often enough to break intermolecular bonds and evaporate them.
PF
Give and proidi the following after and undergoing alpha decay and beta decay
Answers
The products of the alpha decay of radium-226 and the beta decay of carbon-14 are radon-222 and nitrogen-14, respectively.
The alpha decay of radium-226 results in the emission of an alpha particle, which is a helium nucleus consisting of two protons and two neutrons.
Therefore, the product of the alpha decay of radium-226 is radon-222:
Ra-226 → Rn-222 + alpha particle
On the other hand, In the case of carbon-14, beta minus decay occurs, in which a neutron is converted into a proton, and an electron and an antineutrino are emitted.
So carbon-14 becomes nitrogen-14:
C-14 → N-14 + beta particle
To know more about alpha decay, here
brainly.com/question/27870937
#SPJ1
--The complete Question is, What is the product of the alpha decay of radium-226 and the beta decay of carbon-14?--
Your little sister asks you a scientific question: "Does chocolate milk come from brown cows?" In order to answer the question, you decide to form a hypothesis.
Explain whether or not the following statements are effective hypotheses.
i. Brown cows produce chocolate milk.
ii. Brown cows never produce chocolate milk.
iii. Brown cows produce white milk.
Answers
A hypothesis is a proposed explanation or prediction based on limited evidence or observations, which can be tested through further investigation or experimentation. It should be specific, testable, and based on existing knowledge.
Now, let's evaluate each statement as a hypothesis:Brown cows produce chocolate milk.This statement can be considered an effective hypothesis as it proposes a relationship between the color of cows and the color of milk they produce. It is specific and testable, as one could observe and analyze the milk produced by brown cows to see if it is indeed chocolate milk. However, based on existing knowledge, we can confidently say that this hypothesis is not accurate, as the color of a cow does not determine the color of the milk it produces.Brown cows never produce chocolate milk.This statement can also be considered an effective hypothesis because it makes a specific claim that can be tested. However, based on existing knowledge, we can say that this hypothesis is not accurate. While the color of a cow does not determine the color of the milk, it is possible for chocolate milk to be produced by adding chocolate syrup or cocoa powder to regular white milk.Brown cows produce white milk.This statement is not an effective hypothesis as it is a general statement that aligns with existing knowledge. It does not propose any specific relationship or prediction to be tested. In the context of this question, the statement is not accurate as milk produced by cows is typically white, regardless of their coat color.For such more question on hypothesis
https://brainly.com/question/606806
#SPJ8
If the molecules in the above illustration react to form NH3 according to the equation N2 3 H2 2 NH3 , the limiting reagent is , the number of NH3 molecules formed is , and the number of molecules in excess is
Answers
Answer:
Follows are the solution to these question:
Explanation:
Given equation:
\(N_2+3H_2 \longrightarrow 2NH_3\)
In this equation:
\(1 N_2\) gives \(= 2NH_3\)
so,
\(3N_2\) gives= \(2 \times 3 = 6 NH_3\)
similarly:
\(3H_2\) gives \(= 2NH_3\)
So, \(6H_2\) gives = \(\frac{2}{3}\times 6=4NH_3\)
Its limited reagent is =\(N_2\)
The amount of \(NH_3\) molecules were formed = 4.
and the amount of \(H_2\) excess molecules are= 1
when molecules colide is the explosion instant or is there a delay if so why is there a delay
Answers
When molecules collide, there is generally a delay before an explosion occurs, if an explosion occurs at all. This is because an explosion requires a chemical reaction to take place, which involves the breaking of chemical bonds and the formation of new bonds. These reactions require a certain amount of energy, which is called the activation energy.
The collision of molecules can provide energy to the system, but it is not always enough to overcome the activation energy needed for a chemical reaction to occur. If the energy of the collision is less than the activation energy, no reaction will occur and there will be no explosion. If the energy of the collision is equal to or greater than the activation energy, a chemical reaction may occur and an explosion may result.
The time delay between the collision of molecules and an explosion occurring depends on the nature of the molecules and the conditions under which the collision occurs. Some chemical reactions are very fast and can occur almost instantly, while others may take longer to reach the activation energy and proceed to completion.
It's worth noting that not all collisions between molecules result in an explosion. In many cases, the collision may simply transfer energy from one molecule to another without leading to a chemical reaction.
How many carbon and hydrogen atoms are in KNO3?
Answers
Answer: 3 mol oxygen.
Explanation: Because by definition you have 1 mol of potassium nitrate. Potassium nitrate in this quantity comprises 1 mol K, 1 mol N,