Question 6 of 20 30.00 mL of a H2SO4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 39.41 mL of a 0.1347 M NaOH solution. What is the concentration of the H2SO4 solution
Answers
The concentration of the H₂SO₄ solution if 30.00 mL of a H₂SO₄ solution with an unknown concentration was titrated to a phenolphthalein endpoint with 39.41 mL of a 0.1347 M NaOH solution is 0.0884 M.
To calculate the concentration of the H₂SO₄ solution, we must write the balanced equation for the reaction of sulfuric acid (H₂SO₄) and sodium hydroxide (NaOH):
H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O
First, let's calculate the number of moles of NaOH used:
Volume of NaOH solution = 39.41 mL = 0.03941 L
Concentration of NaOH solution = 0.1347 M
Number of moles of NaOH used = concentration × volume = 0.1347 M × 0.03941 L = 0.005304 mol
Now, we can use the balanced equation to determine the number of moles of H₂SO₄. From the equation, we can see that 1 mole of H₂SO₄ reacts with 2 moles of NaOH.
Therefore, number of moles of H₂SO₄ = 0.5 × number of moles of NaOH
= 0.5 × 0.005304 mol = 0.002652 mol
Finally, we can calculate the concentration of the H₂SO₄ solution:
Volume of H₂SO₄ solution = 30.00 mL = 0.03 L
Number of moles of H₂SO₄ = 0.002652 mol
Concentration of H₂SO₄ solution = number of moles/volume = 0.002652 mol/0.03 L = 0.0884 M
Therefore, the concentration of the H₂SO₄ solution is 0.0884 M.
Learn more about concentration: https://brainly.com/question/32222621
#SPJ11
Related Questions
CAN SOMEONE ANSWER ME!! 5-6

Answers
Answer:
As the axial tilt increases, then the seasonal contrast increases so that winters are colder and summers are warmer in both hemispheres. The northern hemisphere is tipped away from the Sun, producing short days and a low sun angle. What kind of effect does the earth's tilt and subsequent seasons have on our length of daylight (defined as sunrise to sunset). Over the equator, the answer is not much.
A hot air balloonist puts 52000 L of air into their balloon at 500 Celsius and 975 atm. When they heat
the air to 750 celsius what is the final volume (in cm^3) in the balloon?
Answers
Answer: The final volume in the balloon is \(68.818cm^3\)
Explanation:
Charles' Law states that volume is directly proportional to the temperature of the gas at constant pressure and number of moles of gas.
Mathematically,
\(\text{Volume}\propto \text{Temperature}\)
Or,
\(\frac{V_1}{T_1}=\frac{V_2}{T_2}\) (At constant pressure and number of moles)
\(V_1\) = initial volume = 52000 L
\(V_2\) = final volume = ?
\(T_1\) = initial temperature = \(500^0C=(500+273)K=773 K\)
\(T_2\) = final temperature = \(750^0C=(750+273)K=1023 K\)
\(\frac{52000}{773}=\frac{V_2}{1023}\)
\(V_2=68818L=68.818ml=68.818cm^3\) \((1L=1000ml=1000cm^3)\)
Thus final volume in the balloon is \(68.818cm^3\)
The solid has a mass of 180 g. What is the density of the solid? Show your work. Be sure to use correct units of measurement. HELP NEEDED ASAP!!!!
Answers
Answer:
I'm telling the teacher on u man stop cheatingA delivery driver's car has a mass of 1500 kg and
is moving at 5 m/s.
The car is unloaded and travels at 10 m/s. If the
truck has the same momentum in each case,
what is the mass of the empty truck?
75 kg
Answers
Answer:
should be half wich is 750
Explanation:
The value of the rate constant at 302°c is 2. 45 × 10-4 l/mol s and at 508°c the rate constant is 0. 0965 l/mol s. the value of r is 8. 3145 j/k mol. Calculate the activation energy for this reaction
Answers
The activation energy for this reaction is - 55.5 kJ/mol
Calculation,
Given data,
First temperature \(T_{1}\) = 302°C = 302+273 = 575 K
Second temperature \(T_{2}\) = 508°C = 508+273 = 781 K
rate constant at 302°C = 2. 45 × \(10^{-4}\) lit/mol s
rate constant at 508°C = 0. 0965 lit/mol s.
Value of universal gas constant = 8.3145 J/k mol.
Apply Arrhenius equation,
㏒\(K_{1} /K_{2}\) = \(E_{a}/2.203R\) [1/ 575 K - 1/781 K]
㏒2. 45×\(10^{-4}\) lit/mol s/0.0965 lit/mol s = \(E_{a}\) /2.303×8.3145 J/k mol[781-575/575K×781 K]
\(E_{a}\) = - 55.5 kJ/mol
learn more about activation energy ,
https://brainly.com/question/11334504
#SPJ4
what are the signs of the enthalpic and entropic terms for formation of secondary structure motifs (e.g., α-helices or β-sheets)?
Answers
The enthalpic term is favorable, while the entropic term is unfavorable for the formation of secondary structure motifs.
Are the enthalpic and entropic terms favorable for secondary structure motif formation?In the context of secondary structure motifs like α-helices or β-sheets, the enthalpic term refers to the energy changes associated with the formation of hydrogen bonds and other stabilizing interactions within the motif.
The enthalpic term is generally favorable for the formation of secondary structures since the establishment of these interactions contributes to the stability and structural integrity of the motif.
On the other hand, the entropic term relates to the changes in molecular freedom or disorder upon the formation of secondary structure motifs.
When a protein adopts a specific secondary structure, there is a reduction in conformational flexibility, resulting in a decrease in entropy.
This entropic term is typically unfavorable for the formation of secondary structures since it restricts the range of accessible conformations for the protein.
Overall, the enthalpic term, driven by favorable interactions, promotes the formation of secondary structure motifs, while the entropic term, driven by reduced conformational flexibility, poses an unfavorable contribution to the process.
Learn more about enthalpic term
brainly.com/question/30516012
#SPJ11
based on the wavelength that the cobalt(ii) chloride solution absorbed most strongly, what color light did the copper(ii) sulfate solution absorb most strongly?
Answers
Copper(II) sulfate is pale blue (cyan) because it absorbs light in the red region of the spectrum.
What is Spectrophotometry ?Spectrophotometry is the branch of electromagnetic spectroscopy that deals with the quantitative measurement of reflectance or transmission properties of materials as a function of wavelength.
A method of measuring how much light a chemical absorbs by measuring the intensity of light as it passes through a sample solution. The basic principle behind spectrophotometry is that each compound absorbs or transmits light in a specific wavelength range.
Copper(II) sulfate solution appears blue because it actually absorbs red region of spectrum which is a complementary color of blue.
To know more about Spectrophotometry visit:
https://brainly.com/question/24183759
#SPJ4
Calcium carbonate, when heated, forms calcium oxide and carbon dioxide. 100grams of calcium carbonate will produce 56grams of calcium oxide. How many grams of carbon dioxide will it produce? Show working out
Answers
Answer:
It will produce 44 grams of carbon dioxide
Explanation:
Step 1: Data given
MAss of CaCO3 = 100 grams
Molar mass of CaCO3 = 100.09 g/mol
Mass of CaO produced = 56 grams
Molar mass of CaO = 56.08 g/mol
Step 2: The balanced equation
CaCO3 → CaO + CO2
Step 3: Calculate moles CaCO3
Moles CaCO3 = mass CaCO3 / molar mass CaCO3
Moles CaCO3 = 100 grams / 100.09 g/mol
Moles CaCO3 = 1.00 moles
Step 4: Calculate moles CaO
Moles CaO = 56 grams / 56.08 g/mol
Moles CaO = 1.00 moles
Step 5: Calculate moles CO2
For 1 mol CaCO3 we'll have 1 mol 1 mol CaO and 1 mol CO2
Step 6: Calculate mass CO2
Mass CO2 = moles CO2 * molar mass CO2
Mass CO2 = 1.00 moles * 44.0 g/mol
Mass CO2 = 44 grams
It will produce 44 grams of carbon dioxide
At a particular pressure and temperature, nitrogen gas effuses at the rate of 82 ml/s. using the same apparatus at the same temperature and pressure, at what rate will nitrogen dioxide effuse?
Answers
The rate at which the nitrogen dioxide, NO₂ will effuse is 64 mL/s
Graham's law of diffusionThis states that the rate of diffusion of a gas is inversely proportional to the square root of the molar mass i.e
R ∝ 1/ √M
R₁/R₂ = √(M₂/M₁)
Where
R₁ and R₂ are the rates of each gasM₁ and M₂ are the molar mass of each gasHow to determine the rate at which nitrogen dioxide, NO₂ will deffuseRate of N₂ (R₁) = 82 mL/sMolar mass of N₂ (M₁) = 28 g/mol Molar mass of NO₂ (M₂) = 46 g/molRate of NO₂ (R₂) =?Applying the Graham's law of diffusion equation, we have:
R₁/R₂ = √(M₂/M₁)
82 / R₂ = √(46 / 28)
Cross multiply
82 = R₂√(46 / 28)
Divide both sides by √(46 / 28)
R₂ = 82 /√(46 / 28)
R₂ = 64 mL/s
Thus, nitrogen dioxide, NO₂ will effuse at 64 mL/s
Learn more about Graham's law of diffusion:
https://brainly.com/question/14004529
#SPJ4
If you were to reach the location in 316 minutes, what is your average speed in
Kilometers per hour? It would take us 5 hours and 2 minutes to get there at the speed of
8.3 kilometers per hour.
What is your average speed if your frame of reference is the rotation of the Earth.
Explain how you came to this conclusion.
Answers
The average speed is zero if our frame of reference is the rotation of the Earth because the relative distance with respect to the earth is zero.
Average speed = 0.26 km/hr
total time = 316 minutes
distance = speed * time
= 8.3 * 5*1/30 = 8.3 *1/6
= 1.38 km
Average speed = total distance / total time
1.38 km / 5.27
= 0.26 km/hr
Distance is described to be the importance or length of displacement among positions. observe that the gap between two positions is not the same as the distance traveled between them. Distance traveled is the whole period of the path traveled among positions. Distance traveled isn't a vector.
There are three foremost styles of average: imply, median, and mode. each of these strategies works barely otherwise and frequently results in slightly distinct ordinary values. The suggest is the maximum usually used commonly. To get the mean cost, you add up all of the values and divide this general by means of the variety of values.
Learn more about average speed here:-https://brainly.com/question/4931057
#SPJ4
dentify the base in this acid-base reaction:
Upper N a upper O upper H plus upper H upper C l right arrow upper N a upper C l plus upper H subscript 2 upper O.
NaOH
HCl
NaCl
H2O
Answers
Answer:
NaOH
Explanation:
Sodium hydroxide is the base in the acid-base reaction as all other acids and sodium chloride is salt and water is neutral.
What is a base?According to the Arrhenius concept, base is defined as a substance which yields hydroxyl ions on dissociation.These ions react with the hydrogen ions of acids to produce salt in an acid-base reaction.
Bases have a pH higher than seven as they yield hydroxyl ions on dissociation.They are soapy in touch and have a bitter taste.According to the Lowry-Bronsted concept, base is defined as a substance which accepts protons .Base react violently with acids to produce salts .Aqueous solutions of bases can be used to conduct electricity .They can also be used as indicators in acid-base titrations.
They are used in the manufacture of soaps,paper, bleaching powder.Calcium hydroxide ,a base is used to clean sulfur dioxide gas while magnesium hydroxide can be used as an antacid to cure acidity.
Learn more about base,here:
https://brainly.com/question/12445440
#SPJ3
Which two carbon cycle components have essentially the same amount of carbon exchange in gigatons (Gt) per year
Answers
The terrestrial biosphere (land plants and ecosystems) and the ocean are the two carbon cycle components that have essentially the same amount of carbon exchange in gigatons (Gt) per year.
Through activities like photosynthesis, respiration, and carbon uptake/storage, both the terrestrial biosphere and the ocean contribute to the exchange of carbon with the atmosphere.
The movement of carbon between these two parts aids in preserving the equilibrium of carbon in the Earth's system. Therefore, the terrestrial biosphere (land plants and ecosystems) and the ocean are the two carbon cycle components that have essentially the same amount of carbon exchange in gigatons (Gt) per year.
Learn more about carbon cycle, here:
https://brainly.com/question/22741334
#SPJ4
What do all of the cells have in common?
A) cell membrane B) endoplasmic reticulum C) flagellum D) nucleus
Answers
PLEASE ANSWER QUICKLY ASAP
READ QUESTIONS CAREFULLY
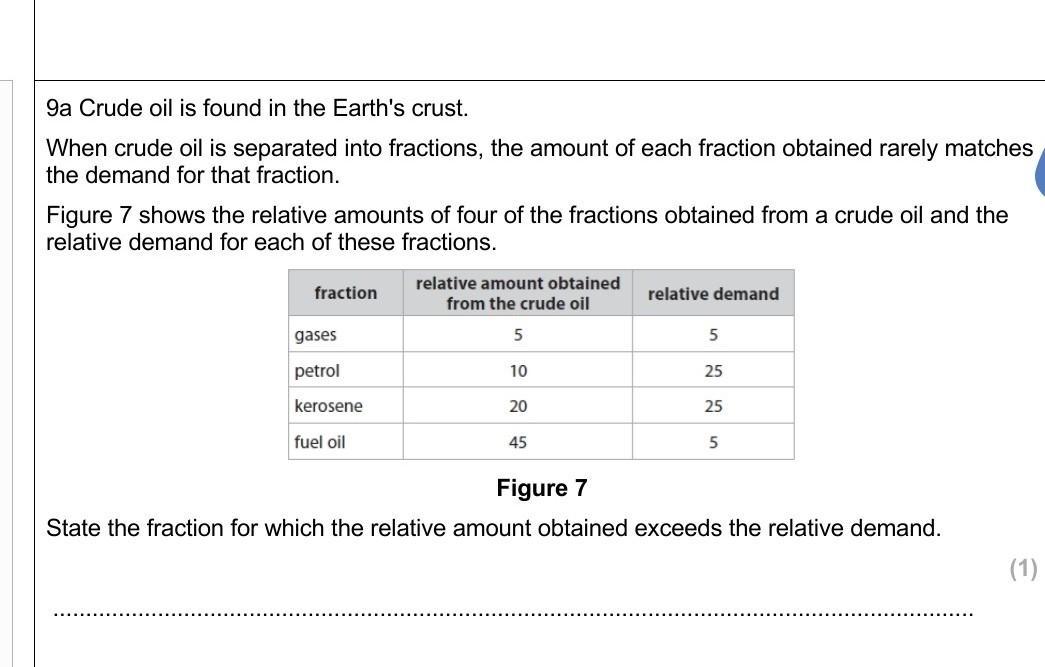
Answers
Answer:
45/9 or 5
Explanation:
the oil which relative amount is greater than demand is fuel oil, whose fraction is 45/9
Line notation: Sn (s)|Zn 2+ (aq,0.022M) || Ag+ (2.7M)| Ag(s) E° cell = 0.94 V
(a) Write the balanced equation.
(b) What is the cathode?
(c) Calculate E cell of the line notation above.
(d) Compare E° cell with E cell calculated from part (c). Then explain if you answer make sense in terms of Le Chatelier's principle.
Answers
(a) The balanced equation for the given cell notation would be:Sn(s) + 2Ag+(aq) → Sn2+(aq) + 2Ag(s)
(b) The cathode would be Ag+(aq), and Ag(s) would gain electrons to produce Ag atoms.
c)The E cell for the given cell notation can be calculated as follows:E° cell = 0.94 V
We know that the E° cell is the standard cell potential when the concentrations of all the reactants and products are 1 M. We can use the Nernst equation to calculate the cell potential for non-standard conditions.
The Nernst equation is given by:Ecell = E°cell - (0.0592/n)logQ,
where n is the number of electrons involved in the reaction and Q is the reaction quotient.Q can be calculated using the concentrations of the species involved in the reaction.
Here, we have:Q = [Sn2+]/[Ag+]² = 0.022/[2.7]² = 0.003We know that n = 2 (from the balanced equation).Plugging in the values, we get:
Ecell = 0.94 - (0.0592/2)log0.003
Ecell = 0.94 + (0.0592/2)(2.5229)
Ecell = 0.94 - 0.0748Ecell = 0.8652 V(d)
The E° cell is 0.94 V, and the calculated Ecell is 0.8652 V.
Since the calculated Ecell is less than E° cell, the reaction is shifted towards the reactants, as per Le Chatelier's principle.
As the reaction shifts towards the reactants, the concentration of Sn2+ increases while the concentration of Ag+ decreases.
The reaction quotient Q increases and the value inside the log in the Nernst equation becomes smaller, reducing the value of Ecell. Therefore, the calculated value for Ecell makes sense in terms of Le Chatelier's principle.
To know more about cell notation refer here :
https://brainly.com/question/31977987#
#SPJ11
The diagrams below are illustrations of some farm tools. Study them carefully and use
them to answer the questions that follow.
1)
iii)
M
Die
N
P
T.
Q
Identify each of the tools labelled M, N, P and Q.
Mention one use each of the tools labelled M, N, P and Q.
[4 marks]
[4 marks]
State two precautions that must be taken when using the labelled P. [2 marks].
Answers
According to the information we can infer that these tools are: P.aspersor, Q. sword, M. manual drill, N. blind. According to the above, these tools are used to build and sprinkle crops.
What tools do we see in the image?According to the image we can infer that the different tools are:
P. sprinkler.Q. sword.M. hand drill.N. blind.On the other hand, the functions of these tools are:
P. apply substances on crops.Q. Cut crops.M. Make holes.N. Make cuts.The precautions that we must take with these tools (P) are:
Good handling.Use personal protection elements.Note: This question is incomplete. Here is the complete information:
Attached image
Learn more about tools in: https://brainly.com/question/31719557
#SPJ1

I would like to know if question number 3 is rite

Answers
The balanced reaction of this chemical reaction is as follows :
\(2HClO_4(aq)\text{ + Ba}(OH)_2(aq)\text{ }\Rightarrow Ba(ClO_4)_2(aq)+2H_2O(l)\text{ }\)• As we can see, this is a double-displament reaction
Which two words apply to the substance copper sulphate?
Please give 1 answer.
A.
solid, compound
В.
gas, element
C
solid, mixture
D.
gas, compound
Answers
Answer: A. solid, compound
Explanation:
Because copper sulphate is a compound and it is a blue solid powder
Hope that help :)
Suppose cobalt-60 undergoes a type of radioactive decay that does not
change the identity of the isotope. which type of decay did the isotope
undergo?
a. delta
b. gamma
c. alpha
d. beta
Answers
Which has more thermal energy, an ice berg or a hot cup of coffee?
Answers
Answer: an iceberg
Explanation: the reason for this is because it has much more mass then a hot cup of coffee even the the temputure of coffee is much warmer
How many atoms are in 1g of Hg
Answers
Answer:
7.5275 x 10^21
Explanation:
Answer:
\(\boxed {\boxed {\sf 3 \times 10^{21} \ atoms \ Hg}}\)
Explanation:
We are asked to find how many atoms are in 1 gram of mercury.
1. Convert Grams to MolesFirst, we convert grams to moles. We use the molar mass or the mass of 1 mole of a substance. These values are equivalent to the atomic masses on the Periodic Table, but the units are grams per mole instead of atomic mass units.
Look up mercury's molar mass.
Hg: 200.59 g/molWe convert using dimensional analysis, so we create a ratio using the molar mass.
\(\frac { 200.59 \ g \ Hg}{ 1 \ mol \ Hg}\)
We are converting 1 gram of mercury to moles, so we multiply the ratio by this value.
\(1 \ g \ Hg *\frac { 200.59 \ g \ Hg}{ 1 \ mol \ Hg}\)
Flip the ratio so the units of grams of mercury cancel out.
\(1 \ g \ Hg *\frac{ 1 \ mol \ Hg} { 200.59 \ g \ Hg}\)
\(1 *\frac{ 1 \ mol \ Hg} { 200.59}\)
\(\frac{ 1} { 200.59} \ mol \ Hg\)
\(0.004985293385 \ mol \ Hg\)
2. Convert Moles to AtomsNext, we convert moles to atoms. We use Avogadro's Number or 6.022 × 10²³. This is the number of particles (atoms, molecules, formula units) in 1 mole of a substance. In this case, the particles are atoms of mercury.
We will use dimensional analysis and set up another ratio.
\(\frac {6.022 \times 10^{23} \ atoms \ Hg}{ 1 \ mol \ Hg}\)
Multiply by the number of moles we calculated.
\(0.004985293385 \ mol \ Hg * \frac {6.022 \times 10^{23} \ atoms \ Hg}{ 1 \ mol \ Hg}\)
The units of moles of mercury cancel.
\(0.004985293385 * \frac {6.022 \times 10^{23} \ atoms \ Hg}{ 1 }\)
\(3.00214368 \times 10^{21} \ atoms \ Hg\)
3. RoundThe original measurement of grams has 1 significant figures, so our answer must have the same. For the number we calculated, that is the ones place. The 0 in the tenths place tells us to leave the 3 in the ones place.
\(3 \times 10^{21} \ atoms \ Hg\)
There are approximately 3×10²¹ atoms of mercury in 1 gram of mercury.
How would I balance this?

Answers
The balance chemical reaction is as below:
4Fe (s) + 3O₂ (g) ⇒ 2Fe₂O₃ (s)
What is balance chemical reaction ?The term balanced chemical equation is defined when the number of the atoms involved in the reactants side is exactly equal to the number of atoms in the products side.
The most important thing about balancing chemical equations is to satisfy the law of conservation of mass, This states that “the total mass of the products is exactly equal to the total mass of all the reactants”
Thus, The balance chemical reaction is as below:
4Fe (s) + 3O₂ (g) ⇒ 2Fe₂O₃ (s)
To learn more about balance chemical reaction, follow the link;
https://brainly.com/question/30230799
#SPJ1
In terms of the kinetic molecular theory, in what ways are liquids similar to solids? in what ways are liquids different from solids?
Answers
Answer:
particles in liquids have greater kinetic energy than particles in solids
A magnesium ion, Mg2+, hasA) 12 protons and 13 electrons. D) 24 protons and 22 electrons.B) 24 protons and 26 electrons. E) 12 protons and 14 electrons.C) 12 protons and 10 electrons.
Answers
A magnesium ion, Mg2+, has correct answer is option E) 12 protons and 14 electrons.
The correct answer is option E) 12 protons and 14 electrons. This is because the atomic number of magnesium, which is the number of protons in its nucleus, is 12. When it loses two electrons to become an ion, it still has 12 protons but now only 10 electrons. Therefore, the charge on the ion is 2+ (written as Mg2+). Options A, B, C, and D have incorrect numbers of protons and electrons for a magnesium ion.
Mg2+, an ion of magnesium, contains 12 protons and 14 electrons. This is so because magnesium has 12 protons, or its atomic number, in its nucleus. It still has 12 protons but only 10 electrons when it loses two electrons to become an ion. As a result, the ion has a 2+ charge, represented by the symbol Mg2+. For a magnesium ion, the protons and electrons in Options A, B, C, and D are in the wrong proportions.
To know more about magnesium ion click here:
https://brainly.com/question/1698012
#SPJ11
Balance the chemical reaction
using an atom inventory.
What is the correct whole
number coefficient for iron(III)
bromide?
[?] FeBr3+ [ ]Na₂S →
]Fe₂S3 + [ ]NaBr
Please help!!
Answers
Answer:
Explanation:Balance equation:
2 FeBr3 + 3 Na2S → Fe2S3 + 6 NaBr
which of the following types of radiation can be blocked with only a sheet of paper?
beta decay, gamma decay, they are equally dangerous, alpha decay
Answers
Answer:
Alpha decay
Explanation:
Alpha decay can be blocked with only a sheet of paper.
Extra info : -
In general, alpha particles have a very limited ability to penetrate other materials. In other words, these particles of ionizing radiation can be blocked by a sheet of paper, skin, or even a few inches of air.
Hello there!
Alpha decay can be blocked with only a sheet of paper.Gamma rays can only be blocked with something thick and dense.Beta rays can be blocked with something less dense, but thicker than paper.Hope this helps. Let me know if you have any questions.
~GracefulGirlie :)
Good luck on your assignment.
how are reactions between aldehydes and nucleophiels fundamentally different than reactions between acyl chlorides and nucleophiles
Answers
The main difference between reactions with aldehydes and acyl chlorides is the reactivity and range of nucleophiles that can be used.
The reactions between aldehydes and nucleophiles are fundamentally different than reactions between acyl chlorides and nucleophiles in several ways. Aldehydes are less reactive than acyl chlorides due to the absence of the electron-withdrawing effect of the chlorine atom in acyl chlorides. Therefore, reactions with aldehydes are typically slower and require more reactive nucleophiles or higher temperatures. Additionally, aldehydes can undergo reduction reactions to form primary alcohols, whereas acyl chlorides cannot. In contrast, reactions with acyl chlorides are much more reactive due to the electron-withdrawing effect of the chlorine atom, resulting in faster reactions and a wider range of nucleophiles that can be used. Additionally, acyl chlorides cannot undergo reduction reactions to form primary alcohols.
Depending on how the atoms are arranged in their chemical structure, aldehydes and ketones can exist in both cyclic and linear forms. Cyclic aldehydes and cyclic ketones are both feasible; cyclic aldehydes like cyclohexanol and cyclic ketones like cyclohexanone are examples of such molecules. Aldehydes and ketones are two types of organic compounds that belong to the class of compounds known as carbonyl compounds.
Learn more about aldehydes here
https://brainly.com/question/30665943
#SPJ11
How can you show using Pauli's exclusion principle that p sub shell can have only 6 electrons?

Answers
where l = subshell value.
"l"values of subshell are.
s = 0.
p = 1.
d = 2.
f = 3.
So in p orbital we have 6 electrons.
a can of cola contains about 39 grams of sucrose, c12h22o11. how many moles of sucrose does this represent?
Answers
Therefore, there are approximately 0.1135 moles of sucrose in a can of cola.
Sucrose, also known as table sugar, has the chemical formula \(C_{12}H_{22}O_{11}\). The molar mass of sucrose can be calculated by adding up the atomic masses of each element in the molecule. Carbon, hydrogen, and oxygen are the main elements in sucrose and each have a unique atomic mass. By multiplying the number of atoms of each element by its atomic mass, we get the total molar mass of the molecule, which is 342.34 g/mol. To find the number of moles of sucrose in a sample, we divide the mass of the sample by its molar mass. In this case, 39 g of sucrose represents 0.1135 moles.
\((12*12.01) + (11.16.00) = 342.34 g/mol\)
So the number of moles of sucrose is:
\(\frac{39}{342.34} = 0.1135\)
Learn more about sucrose here
brainly.com/question/28869238
#SPJ4

Why does California has areas that are colder than Nevada even though they are similar in latitude?
O Warm ocean currents travel across California.
O Cold ocean currents travel across California.
O Cold ocean currents travel across Nevada.
Warm ocean currents travel across Nevada
O
A
1
2 3
4.
5 6
Answers
Answer:
Explanation:
The main reason why California has areas that are colder than Nevada, despite being at similar latitudes, is due to the influence of ocean currents. Specifically, California is impacted by a cold ocean current known as the California Current, which flows southward along the western coast of North America, while Nevada is an inland state without access to any major ocean currents.
The California Current brings cool water from the Gulf of Alaska down the coast, resulting in lower temperatures along the California coast, particularly in the northern regions. In contrast, Nevada has a desert climate with high temperatures and low humidity, due to its inland location and lack of major bodies of water.
So, the correct option would be "Cold ocean currents travel across California." (Option 2).