Question 4: Balancing Redox Equations (5 points)
Use the following steps to balance the redox reaction below:
Mg + Au* → Mg²+ + Au
a. Write the oxidation and reduction half-reactions. Make sure each half-reaction
is balanced for number of atoms and charge. (3 points)
b. Multiply each half-reaction by the correct number, in order to balance charges
for the two half-reactions. (1 point)
c. Add the equations and simplify to get a balanced equation. (1 point)
Answers
Reactions are balanced by equating the right and the left side by stoichiometry coefficients. The redox reactions are balanced and corrected by simplifying the numbers.
What is a redox reaction?A redox reaction is a depiction of the oxidation and the reduction of the reaction that shows the loss and the gain of the electrons by the chemical species.
The oxidation half-reaction:
Mg → Mg²⁺ + 2e⁻
The reduction half-reaction:
2Au + 2 e⁻ → 2 Au
The overall balanced reaction is:
Mg(s) + 2Au⁺ (aq) → Mg²⁺ (aq) + 2Au(s)
Therefore, the reaction is balanced by the oxidation and the reduction halves.
Learn more about redox equations here:
https://brainly.com/question/17166814
#SPJ1
Related Questions
Jonathon is conducting an experiment to determine how much precipitate (solid product) will form when combining measured volumes of Aich, and NaOH. According to his calculations the reaction should produce 26.0 grams of solid AKOH), when combined. However, when Jonathon measures the mass of the solid precipitate formed in his experiment, he finds that the experiment actually produced 24.5 grams of Al(OH).
Answers
Jonathon's experiment produced 24.5 grams of Al(OH), which is less than the predicted amount of 26.0 grams of AKOH. The discrepancy could be due to measurement errors, incomplete reaction.
What is discrepancy?
To determine the cause of the discrepancy, Jonathon should first review his experimental procedure and make sure that all measurements and calculations were performed accurately. He should also check that the reactants were mixed thoroughly and that the reaction was allowed to proceed to completion. If any errors or inconsistencies are identified, Jonathon should correct them and repeat the experiment to obtain more accurate results.
If the experimental procedure was carried out correctly and the discrepancy cannot be attributed to measurement errors, Jonathon should consider the possibility of impurities in the reactants. Even small amounts of impurities can affect the outcome of a chemical reaction, so it is important to use high-quality, pure chemicals in experiments whenever possible.
Overall, the most important thing for Jonathon to do in this situation is to carefully review his experimental data and methodology, and to identify any potential sources of error or uncertainty. By doing so, he can improve the accuracy and reliability of his results and draw more meaningful conclusions from his experiment.
To know more about reactants, visit:
https://brainly.com/question/13005466
#SPJ9
Which evelt led to the formation of our solar system?
Answers
Answer:
Scientists believe that the solar system was formed when a cloud of gas and dust in space was disturbed
Explanation: I looked It Up on Google
Write the first step of this elimination using curved arrows to show electron reorganization. Remember that a mechanism step may require more than one curved arrow.
Answers
Answer:
Explanation:
The missing image can be seen below.
From the given information:
The elimination process follows E2 mechanism which is a 2nd order kinetics.
At E2 mechanism, the base attaches with the beta hydrogen while also removing the leaving group in the same process. In the given compound 2-chloro-2-methylpropane, chloride is the leaving group that results in the product; 2-methylprop-1-ene.
The mechanism is seen in the second image,


Shown below is a table with plants and animals with their adaptations.
Using the description of plants and animals in the table above, which biome would all of these organisms would be best adapted to?

Answers
Answer:
Desert
Explanation:
The adaptation shown by the given plants and animals shows that they will adapted to the desert biome.
It is so because, due to high temperature of desert some desert animals like camel have the storage of fat in humps or tails; some animals have large ears such as Jackrabbits, it helps to release body heat and adapt in high temperature; plants have thick water holding tissues to reduce water loss in heat and waxy coating that keeps the plants cooler and reduce moisture loss.
Hence, the correct answer is "Desert".
A bronze bell containing copper and tin is
a. pure substance
b. colloidal suspension
c. a solid solution
d. heterogenous mixture
Answers
Answer:
I will go for a heterogeneous mixture
Explanation:
alloy is basically a mixture
Based on the vibrational difference you observed, state a hypothesis to explain why diatomic molecules in the air, such as oxygen and nitrogen, are not greenhouse gases while those with 3 or more atoms, such as methane, are?
Answers
Answer:
See explanation
Explanation:
The ability of a gas to function as a green house gas depends on its ability to absorb infra red rays. In turn, the absorption of infrared red rays depends on whether or not the molecule is IR active.
The triatomic molecules such as methane and water are IR active. Only IR active molecules can lead to green house effect.
Note that for a molecular vibrational mode to be IR active, the dipole moment of the molecule is changed as the vibration occurs .
2. You water three sunflower plants with salt water. Each plant receives a different concentration
of salt solutions. A fourth plant receives pure water. After a two week period, the height is
measured.
IV the amount of salt in the water
DV the height
CG plant that gets just water
Answers
Answer:
This question is asking to identify the variables in the experiment
Independent variable: different salt concentration
Dependent variable: Height of plants
Control group: Plants that receive pure water
Experimental group: Plants that receive different concentration of the salt water solution
Explanation:
The independent variable is the variable that is manipulated or changed by the experimenter in order to bring about a measurable outcome. In this experiment, the independent variable is the DIFFERENT SALT CONCENTRATION the plants were exposed to.
The dependent variable is the variable that responds to the change or manipulation of the independent variable or the measured variable. In this experiment, the dependent variable is the HEIGHT OF THE PLANTS.
The experimental group refers to the group that receives the experimental treatment. The experimental group in this experiment are the PLANTS THAT RECEIVE DIFFERENT CONCENTRATION OF SALT SOLUTIONS.
The control group does not receive any experimental treatment as the independent variable is unchanged. The control group is the PLANT THAT RECEIVE PURE WATER i.e no salt solution
When does a car no longer have kinetic energy
Answers
the formula is (1/2)mv^2
so m stands for mass and v is for velocity
we know that the cars mass will remain however the velocity will change.
so a car would no longer have kinetic energy when it is at rest (no motion).
Please help meeeeeeeeeeeeeeeeeee

Answers
box pointing from Be= alkali earth metals
middle box= transition metals
box pointing from F= halogens
box on far right= noble gases
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
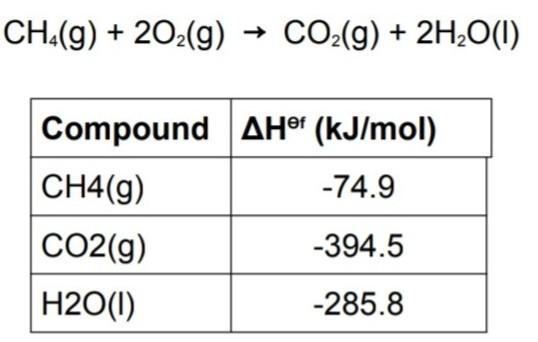
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8
Are pressure and volume directly or inversely proportional
Answers
Pressure and volume can be regarded as the entity that is inversely proportional.
What is the relationship between Pressure and volume?It should be noted that this explanatin an be done using the law in chemistry which is the Boyle's law which states that, for a given amount of gas and constant temperature, the volume is inversely proportional to the pressure.
However the Equal quantities of all gases can be seen to have same number of molecules when subjected to the same temperature and pressure (Avogadro's law).
Learn more about volume at:
https://brainly.com/question/27710307
#SPJ1
What is the hydrogen ion concentration of a solution with pH=7.75 ?
Answers
Answer:
\([H^+]=1.78x10^{-8}M\)
Explanation:
Hello there!
In this case, according to the given information about the pH, it is firstly necessary for us to remember that the pH is defined as the potential of the hydrogen ions in the solution and the concentration of those ions represents how many of them are present in the solution; in such a way, it is possible for us use:
\(pH=-log([H^+])\)
Whereas the concentration of hydrogen ions can be calculated as follows:
\([H^+]=10^{-pH}\)
So we plug in the given pH to obtain:
\([H^+]=10^{-7.75}=1.78x10^{-8}M\)
Regards!
May someone help me please ASAP !
Answers
Answer:
is composed of radio waves
such as x-rays and gamma rays
microwave and radio waves
if am not mistakes this might help
Order the following elements from smallest to largest

Answers
Answer: F, O, N, C, B
Explanation: The element with the greatest electronegativity will have the smallest radius because it holds the electrons closest to the nucleus. Fluorine is the most electronegative element (it is in the upper right of the periodic table), Francium is the lease (it is the lower left of the periodic table). With this knowledge, you should be able to recognize a trend in the periodic table associated with atomic radius size and electronegativity.
Which chemical equation is balanced?
O A. Fe + O₂ → Fe2O3
O B. 2 Fe + 0₂ - > Fe₂O3
O C. 2 Fe + 3 0₂ Fe2O3
D. 4 Fe + 3 0₂ 2 Fe2O3
Answers
Answer:
F
Explanation:
The balanced chemical equation is option D:
4 Fe + 3 O₂ → 2 Fe₂O₃
This is a balanced equation because:
- There are four iron (Fe) atoms on both the reactant and product sides.
- There are three oxygen (O₂) molecules on both the reactant and product sides.
- The coefficients are the smallest possible integers that make the equation balanced.
What is SO2 shape name?
Answers
Answer:Molecular Formula SO2
Hybridization Type sp2
Bond Angle 119o
Geometry V-Shaped or Bent
Explanation:
hope this helped <3
What is the name of the compound N2O3?Trinitrogen dioxideTrinitrogen dioxygenDinitrogen trioxideDinitrogen trioxygen
Answers
Dinitrogen trioxide
Explanations;From the given question, we are to name the compound N2O3. From the given compound, you can see that there are 3 atoms of oxygen and 2 atoms of hydrogen.
Hence the name of the given compound is Dinitrogen trioxide
Which set of terms best defines what affects kinetic energy and potential energy, respecrively
Answers
In order to find the density of an object, Maria is trying to measure its volume. However, the object does not fit in the
tool she is using. To solve this problem, Maria decides to break apart the object.
Will Maria be able to find density following this method? Why or why not?
O Yes, if she measures the volume and mass of all the pieces of the object, she should be able to calculate density
O Yes, if she measures the volume and mass of one of the pieces of the object, she should be able to calculate
density.
O No, once the object is broken apart and the shape has been altered, it is not possible to calculate the volume of
the whole object to find density.
O No, once the object is broken apart and the shape has been altered, it is not possible to calculate the mass of the
object to find density.
Answers
Answer: She needs to divide the mass by the volume to find the density of the object.
Explanation:
Explain how the ionic bond is formed in Lithium Fluoride.
Answers
( I underlined the important part the rest is extra info that can help you understand)
Answer
Lithium is a metal (Alkali metal) it will donate one of its electrons to fluoride so then it becomes positively charged (cation).
(atoms are neutral because protons (p+) and electrons (e-) are equal taking one electron from an atom will make it positive)
Flouride is a nonmetal so it will accept the electron from lithium so then it becomes negatively charged (anion)
lithium is in group 1 so its charge is +1
Flouride is in group 17 so its charge is -1
both cancel each other so there are no subscripts
separate the precipitate formed when aqueous silver nitrate is added to
aqueous sodium chloride.
Answers
This reaction produces a white precipitate. It is silver chloride:
\(AgNO_{3} + NaCl\) → \(AgCl\)↓ \(+\) \(Na^{+} + NO^{-} _{3}\)
Relations to my budget

Answers
When there is an increase in an activity, like sales or manufacturing, the overall amount of an expense, known as a fixed expense, does not change.
Thus, Normal definitions typically include the phrase within a relevant or appropriate range of activity since a change is likely to take place at fixed expense either an exceptionally high or low volume or expense.
Of course, the rent will probably need to adjust if sales quadruple or fall to 20% of the average level. However, as the extreme circumstances are outside of the relevant range for short-term analysis, the current rent of $2,000 is regarded as a fixed expense.)
The following are some instances of costs that are probably set within a fair range of retail sales, The yearly pay for the shop manager.
Thus, When there is an increase in an activity, like sales or manufacturing, the overall amount of an expense, known as a fixed expense, does not change.
Learn more about Expenses, refer to the link:
https://brainly.com/question/29850561
#SPJ1
densityy of a cube that is 2" and weighs 444.5 g
Answers
The density (in g/cm³) of a cube of metal that is 2 inches on a side and weighs 444.5 grams is 3.4 g/cm³ (1st option)
How do i determine the density of the metal?First, we shall obtain the volume of the metal. Details below:
Length (L) = 2 in = 2 × 2.54 = 5.08 cmVolume =?Volume = L³
Volume = 5.08³
Volume = 131.1 cm³
Finally, we shall obtain the density of the metal. Details below:
Volume of metal = 131.1 cm³ Mass of metal = 444.5 gramsDensity of metal = ?Density = mass / volume
Density of metal = 444.5 / 131.1
Density of metal = 3.4 g/cm³
Thus, we can conclude from the above calculation that the density of the metal is 3.4 g/cm³ (1st option)
Learn more about density:
https://brainly.com/question/13275926
#SPJ1
Complete question:
What is the density (in g/cm³) of a cube of metal that is 2 inches on a side and weighs 444.5 grams?
3.4 g/cm³
8.89 g/cm³
55.56 g/cm³
0.180 g/cm³
Write the chemical formula for this molecule

Answers
The chemical formula for the molecule you provided is C2H5Cl.
In the molecule, the central atom is carbon (C), which is bonded to two hydrogen atoms (H) and one chlorine atom (Cl). The carbon atom forms single bonds with each of the hydrogen and chlorine atoms, resulting in a linear structure.
To write the chemical formula, we start by indicating the number of atoms of each element present in the molecule. In this case, there are two carbon atoms (C2), five hydrogen atoms (H5), and one chlorine atom (Cl1).
Next, we write the symbols for the elements in the order of their appearance. The formula is typically written with the carbon atom first, followed by hydrogen, and then any other elements in alphabetical order. Therefore, the chemical formula for the molecule is C2H5Cl.
The subscripts in the formula indicate the number of atoms of each element in the molecule. In this case, there are two carbon atoms, five hydrogen atoms, and one chlorine atom.
It's important to note that the formula represents the simplest ratio of atoms in the molecule. It does not provide information about the spatial arrangement or bonding pattern of the atoms. Additional structural information, such as the arrangement of atoms in space, would require a more detailed representation, such as a Lewis structure or a three-dimensional model.
for more questions on chemical formula
https://brainly.com/question/21393201
#SPJ8
What determines the change of an ion formed by an atom
Answers
Answer:
Ions are formed when the number of protons in an atom does not equal the number of electrons. If more protons are present, the ion is positive and is known as a cation; if more electrons are present, the ion is negative and referred to as an anion. Ions are highly reactive species.
Explanation:
Answer:
An atom becomes charged when the number of protons does not equal the number of electrons. For example, if an element has six protons but only five electrons, the net charge of the element is +1. Conversely, if an element has six protons but seven electrons, then the net charge of the element is -1.
Explanation:
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
You are on a field trip to a nearby lake for biology class and want to perform a quick analysis of the water’s approximate pH level. Which pH measurement system would you use and why?
Answers
Consider the following reaction:
2CH4(g)⇌C2H2(g)+3H2(g)
The reaction of CH4 is carried out at some temperature with an initial concentration of [CH4]=0.092M. At equilibrium, the concentration of H2 is 0.014 M.
Find the equilibrium constant at this temperature.
Answers
The equilibrium constant at this temperature is Kc= 4.17 x 10⁻⁶.
What is equilibrium?Since the equilibrium constant depends on the equilibrium concentration of both the reactants and the products of the chemical reaction.
Balanced reaction equation
2CH₄(g)⇌C₂H₂(g)+3H₂(g)
The initial concentration of the CH₄ = 0.093 M
The equilibrium concentration of the H = 0.017 M
Equilibrium constant = ?
Let's make the ice table
2CH₄(g) ⇌ C₂H₂(g) + 3H₂(g)
0.093 M 0 0
-2x +x +3x
0.093-2x x 0.017 M
3x = 0.017 M
Therefore, x =0.017 M /3 = 0.00567 M
Therefore, the equilibrium concentration of CH₄ =
0.093 M – 2x = 0.093 M – (2 x 0.00567 M) = 0.0817 M
Equilibrium concentration of the C₂H₄ = x = 0.00567 M
Let's write the equilibrium constant expression
Kc= [C₂H₄[H2]³/[CH₄]²
Let's put the values in the formula
Kc= [0.00567][0.017]³/[0.0817]²
Kc= 4.17 x 10⁻⁶
Therefore, the equilibrium constant is 4.17 x 10⁻⁶.
To learn more about equilibrium constant, refer to the link:
https://brainly.com/question/12971169
#SPJ9
Which has the greatest mass? 1 mole Ca 1 mole CO 1 mole O2 1 mole CH4 1 mole NO
Answers
Answer: 1 mole of \(Ca\) has the greatest mass.
Explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP , contains avogadro's number \((6.023\times 10^{23})\) of particles and weighs equal to the molecular mass of the substance.
1 mole of \(Ca\) has a mass of 40 g.
1 mole of \(CO\) has a mass of 28 g
1 mole of \(O_2\) has a mass of 32 g
1 mole of \(CH_4\) has a mass of 16 g.
1 mole of \(NO\) has a mass of 30 g.
Thus the greatest mass is of 1 mole of \(Ca\)