Protons and neutrons have opposite, but equal magnitude, charges. An atom contains the same number of protons and electrons Neutrons and electrons are found in the nucleus of an atom. Protons have about the same mass as electrons. Electrons make up most of the mass of an atom. which one is true about subatomic particles
Answers
Answer:
the true statement is an atom contains the same number of electron and protonExplanation:
let us see the behaviour of the sub atomic particles
nutrons and protons found in the nucleus of an atoman atom have the same number of electron and proton but they have different chargenutron is chargeless particlemost mass of the atom concentrated in the nucleus of an atomelectrons have almost 0 mass from an atommass of proton and mass of nutron are equvalent or almost equalthere are many properties of subatomic particles i have listed some of them above .
I think it is help ful for youRelated Questions
I NEED THIS DONE TODAY !!!!!!!!Electromagnetic Spectrum Lab Report
Destructions: In this virtual lab, you will use a virtual spectrometer to analyze astronomical
bodies in space. Record your hypothesis and spectrometric recular in the lab report below. You
will submit your completed report to your butructor.
Name and Title:
Include your name, instru
1
and name of lab.
Objectives (1):
In your own words, what is the purpose of this lab?
Hypothesis:
In this section, please include the predictions you developed during your lab activity. These
statements reflect your predicted outcomes for the experiment.
Procedure:
The materials and procedures are listed in your virtual lab. You do not need to repeat them here.
However, you should note if you experienced any errors or other factors that might affect your
outcome. Using your summary questions at the end of your virtual lab activity, please clearly
define the dependent and independent variables of the experiment.
Data:
Record the elements present in each unknown astronomical object. Be sure to indicate "yes" or
"no" for each element.
Hydrogen Helium Lithium Sodiam Carbon
Moon One
Moon Two
Planet One
Planet Two
Nitrogen
Conclusion:
Your conclusion will inchade a summary of the lab results and an interpretation of the results.
Please answer all questions in complete sentences using your own words.
1. Using two to three sentences, summarize what you investigated and observed in this lab
2. Astronomers use a wide variety of technology to explore space and the electromagnetic
spectrum; why do you believe it is essential to use many types of equipment when
studying space?
3. If carbon was the most common element found in the moons and planets, what element is
missing that would make them splat to Earth? Explain why. (Hint: Think about the
carbon cycle)
4.
We know that the electromagnetic spectrum uses wavelengths and frequencies to
determine a lot about outer space. How does it help us find out the make-up of stars?
5. Why might it be useful to determine the elements that a planet or moon is made up of?
PLEASE MAKE SURE YOU ANSWER THE HYPOTHESIS AND PROCEDURE QUESTION!!!!
Answers
Below contains the complete lab report on electromagnetic spectrum
The Lab ReportName: [Your Name]
Title: Electromagnetic Spectrum Lab Report
Instructor: [Instructor's Name]
Objectives:
The purpose of this lab is to analyze the elemental composition of different astronomical bodies using a virtual spectrometer and understand the importance of the electromagnetic spectrum in astronomical research.
Hypothesis:
I predict that the moons and planets will have varying compositions of elements, with hydrogen and helium being more common in gaseous bodies and heavier elements like carbon and nitrogen more common in rocky bodies.
Dependent variable: Presence of elements in astronomical bodies
Independent variable: Astronomical bodies (Moon One, Moon Two, Planet One, Planet Two)
Data:
[Please input your data for each object as per your virtual lab results]
Conclusion:
In this lab, I investigated the elemental composition of four different astronomical bodies using a virtual spectrometer and observed the presence or absence of various elements.
It is essential to use many types of equipment when studying space because different instruments can detect and analyze different aspects of the electromagnetic spectrum, providing a comprehensive understanding of the universe.
To make these moons and planets similar to Earth, oxygen would need to be present as it is a vital component of the carbon cycle and essential for life as we know it.
The electromagnetic spectrum helps us find out the makeup of stars by analyzing the emitted light, which contains information about the elements and their abundance within the star.
Determining the elements that a planet or moon is made up of helps us understand their formation, potential for life, and possible resources for future exploration or colonization.
Read more about Electromagnetic Spectrum Lab Report here:
https://brainly.com/question/30699255
#SPJ1
Sulfur trioxide reacts with water to form sulfuric acid according to the following reaction: SO₃ + H₂O → H₂SO₄ Given the atomic mass of hydrogen is 1 amu, the atomic mass of oxygen is 16 amu, and one molecule of sulfuric acid has a mass of 98 amu, what is the atomic mass of sulfur trioxide?
Answers
Explanation:
The atomic mass of sulfur trioxide can be calculated as follows:
1 molecule of sulfuric acid has a mass of 98 amu, and it is composed of 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms. So, the mass of hydrogen and oxygen atoms in 1 molecule of sulfuric acid is (2 * 1 amu) + (4 * 16 amu) = 34 amu.
Therefore, the mass of sulfur in 1 molecule of sulfuric acid is 98 amu - 34 amu = 64 amu.
Since 1 molecule of sulfuric acid is formed from 1 molecule of sulfur trioxide, the atomic mass of sulfur trioxide can be calculated as 64 amu.
Sulfur trioxide reacts with water to form sulfuric acid according to the following reaction: SO₃ + H₂O → H₂SO₄ Given the atomic mass of hydrogen is 1 amu, the atomic mass of oxygen is 16 amu, and one molecule of sulfuric acid has a mass of 98 amu, the atomic mass of sulfur trioxide is 80 amu.
According to the law of conservation of mass, in a reaction, atomic mass of the reactants will be equal to atomic mass of the products if the reaction is balanced and above reaction is balanced. Hence,
Mass of SO₃ + Mass of H₂O = Mass of H₂SO₄
x + 18 = 98
x = 80 amu = Mass of SO₃
Therefore, when Sulfur trioxide reacts with water to form sulfuric acid according to the following reaction: SO₃ + H₂O → H₂SO₄ Given the atomic mass of hydrogen is 1 amu, the atomic mass of oxygen is 16 amu, and one molecule of sulfuric acid has a mass of 98 amu, the atomic mass of sulfur trioxide is 80 amu.
Learn more about atomic mass, here:
https://brainly.com/question/17067547
#SPJ2
STEP 7: LEAD
Initial temperature of metal = 100
✓°C
Initial temperature of water = 22.6
✓ °C
Final temperature of both = 23.3
°C
Subtract to find the temperature
changes for the water and the metal.
AT (water) = 0.7 ✓ °C
AT (metal) = 76.7 ✓ °C

Answers
Answer:
answer is 76
Explanation:
pls mark me brainliest right
Answer:
0.7
76.7
this is for (LEAD) the last one
calculate the number of moles for the quanity 8.06 x 1021 atoms of Pt
Answers
The number of moles for the quanity 8.06 x\(10_{21\) atoms of Pt is approximately 2.61 grams.
To calculate the number of moles for a given quantity of atoms, we can use Avogadro's number and the molar mass of the element. Avogadro's number is 6.022 x 10²³ atoms/mol.
In this case, you have 8.06 x 10²¹ atoms of Pt. To find the number of moles, divide this quantity by Avogadro's number:
8.06 x 10²¹ atoms Pt / 6.022 x 10²³ atoms/mol = 0.0134 mol Pt
So, there are approximately 0.0134 moles of Pt in 8.06 x 10²¹ atoms of Pt.
The molar mass of Pt (platinum) is 195.08 g/mol. To convert the number of moles to grams, multiply the number of moles by the molar mass:
0.0134 mol Pt x 195.08 g/mol = 2.61 g Pt
Therefore, there are approximately 2.61 grams of Pt in 8.06 x10²¹ atoms of Pt.
In summary, the number of moles for the quantity 8.06 x 10²¹ atoms of Pt is approximately 0.0134 moles. This is equivalent to approximately 2.61 grams of Pt. Remember to use Avogadro's number and the molar mass to perform these calculations accurately.
Know more about moles here:
https://brainly.com/question/29367909
#SPJ8
Complete the statements by writing the number from the graph.
The substance is in the gas phase only in region
.
The substance is in both the liquid and the solid phase in region
.
The substance is in only the liquid phase in region
.
The melting point is the temperature at region
.
The boiling point is the temperature at region
Answers
1. Only region 5 has the chemical in the gas phase.
2. Region 2 contains the material in both the liquid and solid phases.
3. Just the liquid phase of the chemical is present in this area.
4. The temperature in region 2 is the melting point.
5. The temperature in area 4 is the boiling point.
A process known as melting or fusing is one in which the phase transitions from a solid to a liquid state while maintaining a constant temperature.Freezing: It is a type of process in which the phase transforms from liquid state to solid state at constant temperature. Evaporation is a process when the phase transitions from a liquid to a gaseous state while maintaining a constant temperature. Condensation : It is a type of process in which the phase shifts from gaseous state to liquid state at constant temperature.A process known as sublimation occurs when a substance goes directly from its solid to its gaseous form at a fixed temperature.
Learn more about boiling point Refer: brainly.com/question/25777663
#SPJ1

Which of the following animals are an example of coevolution? *
Answers
Acacia ant and acacias animals are an example of coevolution
Coevolution is the reciprocal evolutionary change in a set of interacting population over time resulting from the interaction between those population and an example of coevolution that is not characteristics of an arm race but one which provides a mutual benefit to both a plant species and insect is that of the acacia ant and acacia plant and many cases of coevolution can be found between plants and insects
Know more about animal
https://brainly.com/question/28711835
#SPJ1
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
I need help on this organic chemistry question:
------
What are the number of rotational axes and the number of mirror planes for each of these images? (below)
1,1-dichlorocyclopropane
trans-1,2-dichlorocyclopropane
cis-1,2-dichlorocyclopropane
cis-1-bromo-2,3-dichlorocyclopropane (all cis)
1-bromo-1-chlorocyclopropane
trans-1-bromo-2-chlorocyclopropane
cis-1-bromo-2-chlorocyclopropane
Which of these are superimposable?
-------
Thank you!! :)
Answers
When H2S(g) reacts with O2(g) to form H2O(g) and SO2(g), 124 kcal of energy are evolved for each mole of H2S(g) that reacts. Write a balanced equation for the reaction with an energy term in kcal as part of the equation.
Answers
Answer:
2H2S(g) + 3O2(g) → 2H2O(g) + 2SO2(g) + 248kcal
Explanation:
The reaction of the problem occurs as follows:
H2S(g) + O2(g) → H2O(g) + SO2(g)
To balance the reaction we must balance oxygens:
H2S(g) + 3O2(g) → 2H2O(g) + 2SO2(g)
To balance the complete reaction:
2H2S(g) + 3O2(g) → 2H2O(g) + 2SO2(g)
As the energy is evolved, 124kcal are as product in the reactio per mole of H2S. As the balanced reaction contains 2 moles of H2S, the heat evolved is:
124kcal*2 = 248kcal:
2H2S(g) + 3O2(g) → 2H2O(g) + 2SO2(g) + 248kcal
And this is the balanced equation
Explain this method (Froth floatation method)..........

Answers
Answer:
froth flotation is a technique commonly used in the mining industry. In this technique, particles of interest are physically separated from a liquid phase as a result of differences in the ability of air bubbles to selectively adhere to the surface of the particles, based upon their hydrophobicity.
Explanation:
Froth floatation method is commonly used to concentrate sulphide ore such as galena (PbS), zinc blende (ZnS) etc. (ii) In this method, the metaalic ore particles which are perferentially wetted by oil can be separated from gangue. (iii) In this method, the crushed ore is suspended in water and mixed with frothing agent such as pine oil, eucalyptus oil etc. (iv) A small quantity of sodium ethyl xanthate which act as a collector is also added. (v) A froth is generated by blowing air through this mixture. (vi) The collector molecules attach to the ore particles and make them water repellent. (vii) As a result, ore parrticles, wetted by the oil, rise to the surface along with the froth. (viii) The froth is skimmed off and dried to recover the concentration ore. (ix) The gangue particles that are preferentially wetted by water settle at the bottom.
what is the correct name for Sn3(PO4)2
Answers
what happen when a substance change into a new kind of molecules
Answers
Answer:
Chemical Change
Explanation:
Chemical changes occur when bonds are broken and/or formed between molecules or atoms. This means that one substance with a certain set of properties (such as melting point, color, taste, etc) is turned into a different substance with different properties.
PLEASE HELP!!!!
Why are dichotomous keys used?
A. They help with species identification
B. They help us identify everything from plants to minerals based on shared characteristics
C. They help organize a set of species or objects in a neat, easy to read diagram with branches
D. All of the above
Answers
the answer is D all of the above
How many isomers does propane have?
01
02
03
05
Answers
Answer:
A.) 1
Explanation:
Propane only exists in one conformation. It does not have enough carbons to form branches, and there are only hydrogens attached to each carbon. Furthermore, there is no way to twist the carbon or change its orientation (ex. cis- and trans-) to result in a different structure of propane. There is no other way to represent the molecule without drawing a different molecule.
how many particles are in chemicals
Answers
Answer:
6.022 x 10^23 particles
Explanation:
Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the Latin moles, meaning “pile” or “heap.”
One mole is 6.022 x 10^23 of the microscopic particles which make up the substance in question.
Hope this helped! :^)
What is the oxidation state of P in PO43-?
O A. +8
O B. +3
O C. +5
OD. +2
Answers
Answer:
the answer is +5
Explanation:
good luck :)
The processes which are used to convert alkane consists of 5 atoms to insecticide consists of (18) atoms are www.da a) strong heating then rapid quenching then halogenation then polymerization b) polymerization then halogenation then strong heating then rapid quenching c) strong heating then rapid quenching then polymerization then halogenation halogenation then rapid quenching then strong heating then polymerization
Answers
Making a pesticide from a 5-atom alkane to an 18-atom molecule requires a complex process involving many steps and various reactions.
There are many different insecticides on the market, each with a unique chemical composition and mode of operation. The desired end product will determine the exact reactions and conditions needed to make a pesticide from the alkane. In general, making a pesticide from an alkene requires several important steps, including oxidation, halogenation, and cyclization. The first step is the oxidation or halogenation of the alkene to produce a more reactive intermediate.
Therefore, the correct option is D.
Learn more about Pesticides, here:
https://brainly.com/question/30295459
#SPJ1
Your question is incomplete, most probably the complete question is:
The processes which are used to convert alkane consists of 5 atoms to insecticide consists of (18) atoms are
a) strong heating then rapid quenching then halogenation then polymerization
b) polymerization then halogenation then strong heating then rapid quenching
c) strong heating then rapid quenching then polymerization then halogenation halogenation then rapid quenching then strong heating then polymerization
d) The synthesis of an insecticide from an alkane with 5 atoms to a molecule with 18 atoms requires multiple steps and the specific reactions and conditions used will depend on the insecticide being synthesized.
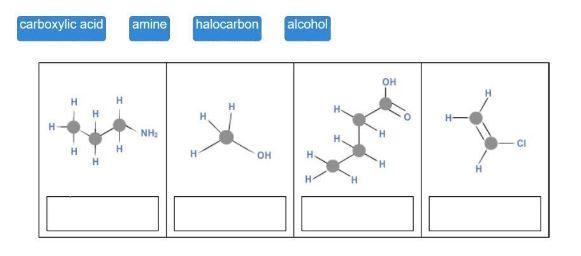
Match each hydrocarbon class to its structure.
4
carboxylic acid
H
H
HT
H
H
H
amine
NH₂
halocarbon
H
OH
alcohol
H.
H
H.
'H
OH
'H
H-
-CI
Answers
The tile's suggested answers include amine, alcohol, carboxyl group, and halocarbon.
Gasoline is it a hydrocarbon?Hydrocarbons are organic substances comprised of hydrogen and carbon, and include petroleum, methane gas, and coal. Alkanes are both a highly combustible chemical and the main source of fuel in the planet. Its uses include diesel, jet fuel, propane, petrol, and petroleum, to name a few.
What makes it a hydrocarbon?The most fundamental category of organic compounds is referred to as a hydrocarbon. As their name implies, they are exclusively made up of the elements hydrogen and carbon. Atoms surround one or more core carbon atoms in hydrocarbon molecules, which are branching or chain-like in shape.
To know more about Hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ1
The complete question is-
Drag each tile to the correct image. Match each hydrocarbon class to its structure. carboxylic acid amine halocarbon alcohol.
Dalton’s completing an investigation in the science lab. He observes that a sample of liquid turns to gas at 135°C. What’s this temperature called?
A.
boiling point
B.
freezing point
C.
melting point
D.
room temperature
E.
standard temperature
.
Answers
Boiling is the process by which a liquid turns into a vapor (gas) when it is heated to its boiling point.
MULTIPLE CHOICE
Question 3
ed
Which elements are transition metals?
Select all that apply.
A
iron
B.
magnesium
с
copper
D
mercury
Answers
Answer:
iron #26, Cu #29, and mercury #80 are transition metals
Explanation:
MULTIPLE CHOICE
Question 3
ed
Which elements are transition metals?
Select all that apply.
A
iron
B.
magnesium
с
copper
D
mercury
look at a periodic table
columns 1,2,13,14,15,16, 17, and 18 are the "representative elements'
columns 3-12 are the transition metals
iron #26, Cu #29, and mercury #80 are transition metals
If powdered platinum metal is used to speed up the following reaction: Cl2(g) 3F2(g) --> 2ClF3(g), what would you classify the platinum as
Answers
Answer:
Catalyst
Explanation:
For the reaction:
\(Cl_2_(_g_)~+~3F_2_(_g_)->2ClF_3_(_g_)\)
We have a main observation: When platinum is added the reaction goes faster. With this in mind, we have to remember the kinetic equilibrium theory. In figure 1, we have an energy diagram. In which we have an specific energy for the reagents and the products. When the reaction takes place, the reaction has to must go through an energy peak. This energy peak is called "activation energy". When platinum is added the activation energy decreases and the reaction can go faster. Therefore, platinum is a "catalyst", a substance with the ability to reduce the activation energy.
I hope it helps!

what is the density of a board dimensions are 5.54 cm x 10.6 cm X 199 cm and whose mass is 28.6kg
Answers
Answer:
\(d=2.44\ g/cm^3\)
Explanation:
Given that,
The dimensions of the board is 5.54 cm x 10.6 cm X 199 cm.
The mass of the board is 28.6 kg.
Since, 1 kg = 1000 grams
28.6 kg = 28.6 × 1000 g = 28600 grams
Density = mass/volume
So,
\(d=\dfrac{28600\ g}{(5.54\times 10.6\times 199)\ cm^3}\\\\d=2.44\ g/cm^3\)
So, the density of a board is \(2.44\ g/cm^3\).
Which is true about the role of the riparian foliage on rivers and streams?
A) increases photosynthesis
B) removes shade
C) decreases current flow
D) provides shade
E) increases the temperature within the system
Answers
Answer:
A) increases photosynthesis
Answer:
D provides shade
Explanation:
I took the test
In what state of matter is the substance in at 200 C and 30 atm
Answers
How is the kinetic energy of the particles of a substance affected during a phase change?
O Kinetic energy increases during exothermic changes and decreases during endothermic changes.
O Kinetic energy decreases during exothermic changes and increases during endothermic changes.
O Kinetic energy does not change, but the potential energy does.
O Kinetic energy changes in the opposite way that the potential energy changes.
Answers
Answer:
O Kinetic energy does not change, but the potential energy does
Calculate the molarity of 0.650 mol of Na2 in 1.50 L of solution.
Molarity:
Answers
Answer: the molarity of 0.650 mol of Na2 in 1.50 L of solution is 0.433 M.
Explanation: the molarity of 0.650 mol of Na2 in 1.50 L of solution is 0.433 M.
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
If a machine has an AMA of 1 what does this tell you about the RF and EF?
Answers
Answer:
Although you might think of a machine as complex system of gears, drive belts and and a motor, the definition physicists use is much simpler. A machine is simply a device that does work, and there are only six different types of simple machines. They include the lever, the pulley, the wheel and axle, the screw, the wedge and the inclined plane.
Explanation:
what is the concentration of a nitric acid solution if 10.0 ml of the solution is neutralized by 3.6 ml of 0.2 m naoh?
Answers
Answer:
The concentration of the nitric acid (HNO3) solution is 72 M.
Explanation:
To determine the concentration of the nitric acid solution, we can use the concept of stoichiometry and the equation of the neutralization reaction between nitric acid (HNO3) and sodium hydroxide (NaOH):
HNO3 + NaOH → NaNO3 + H2O
The balanced equation shows that the molar ratio between HNO3 and NaOH is 1:1. This means that 1 mole of HNO3 reacts with 1 mole of NaOH.
Given:
Volume of HNO3 solution = 10.0 ml
Volume of NaOH solution = 3.6 ml
Molarity of NaOH solution = 0.2 M
To find the concentration of the HNO3 solution, we need to calculate the number of moles of NaOH used in the neutralization reaction:
moles of NaOH = volume of NaOH solution * molarity of NaOH solution
= 3.6 ml * 0.2 M
= 0.72 mmol (millimoles)
Since the molar ratio between HNO3 and NaOH is 1:1, the number of moles of HNO3 in the solution is also 0.72 mmol.
Now, we can calculate the concentration of the HNO3 solution using the formula:
concentration (in M) = moles of solute / volume of solution (in L)
concentration = 0.72 mmol / 0.010 L
= 72 mmol/L
= 72 M
Therefore, the concentration of the nitric acid (HNO3) solution is 72 M.
A hydronium ion:______.a. has the structure H3O. b. is a hydrated hydrogen ion. c. is a hydrated proton.d. is the usual form of one of the dissociation products of water in solution.e. all the answers above are correct.
Answers
Answer:
e. all the answers above are correct.
Explanation:
A hydronium ion:
a. has the structure H₃O⁺. YES, this is the chemical formula of the hydronium ion.
b. is a hydrated hydrogen ion. YES, it is formed according to the equation:
H⁺ + H₂O ⇒ H₃O⁺
c. is a hydrated proton. YES, since proton is the name given to the hydrogen ion
d. is the usual form of one of the dissociation products of water in solution. YES, according to the following equation:
2 H₂O ⇒ H₃O⁺ + OH⁻