pq7 the solubility of lead (ii) iodide in water is 0.62 grams per 1.0l of solution. what is solubility produce constant, ksp, for dissolution
Answers
"The solubility product constant, often denoted as Ksp, is a quantitative measure of the solubility of a sparingly soluble compound in a solvent." It represents the equilibrium constant for the dissolution of the compound into its constituent ions in a saturated solution.
To determine the solubility product constant (Ksp) for the dissolution of lead (II) iodide in water, as follows:
1. Convert the solubility of lead (II) iodide in grams per liter (0.62 g/L) to moles per liter (M) using its molar mass (461.01 g/mol):
(0.62 g/L) / (461.01 g/mol) = 0.00134 M
2. Write the balanced dissolution equation for lead (II) iodide:
PbI₂ (s) ⇌ Pb²⁺ (aq) + 2I⁻ (aq)
3. Determine the equilibrium concentrations of Pb²⁺ and I⁻ ions based on the solubility of lead (II) iodide:
[Pb²⁺] = 0.00134 M
[I⁻] = 2 × 0.00134 M = 0.00268 M
4. Calculate the solubility product constant (Ksp) using the equilibrium concentrations:
Ksp = [Pb²⁺] × [I⁻]² = (0.00134) × (0.00268)² = 9.62 × 10⁻⁹
The solubility product constant (Ksp) for the dissolution of lead (II) iodide in water is approximately 9.62 × 10⁻⁹.
When a sparingly soluble compound dissolves in a solvent, it dissociates into its constituent ions. The equilibrium expression for this dissolution process can be written as follows, taking the general form of the compound AX:
AX(s) ⇌ A+(aq) + X-(aq)
The corresponding equilibrium constant expression is:
Ksp = [A+][X-]
where [A+] represents the concentration of the cation A+ and [X-] represents the concentration of the anion X-. The concentrations are typically expressed in molarity (M).
The solubility product constant, Ksp, is defined as the product of the concentrations of the constituent ions at equilibrium, with each concentration raised to the power of its stoichiometric coefficient. The stoichiometric coefficients are determined by the balanced chemical equation for the dissolution process.
The value of Ksp is specific to a particular compound and is temperature-dependent. It is a constant at a given temperature and represents the maximum amount of solute that can dissolve in a given solvent to form a saturated solution. The higher the value of Ksp, the more soluble the compound is in the solvent.
By knowing the value of Ksp, you can determine the solubility of a compound in a solvent or predict the formation of a precipitate when the ion concentrations exceed the solubility product.
It's worth noting that the solubility product constant, Ksp, is different from the equilibrium constant, Kc, which is associated with reactions in the gas or solution phase and involves the concentrations of all species, not just the ions in the saturated solution.
To learn more about solubility constant product visit: https://brainly.com/question/1419865
#SPJ11
Related Questions
13 how does the pattern of chlorophyll differ between the leaf kept in the dark versus the leaf kept in the light?
Answers
The pattern of chlorophyll in leaves depends on the exposure to light, with leaves kept in the light having a higher and more uniform chlorophyll content compared to those kept in the dark.
The pattern of chlorophyll in leaves differs significantly when comparing a leaf kept in the dark versus a leaf kept in the light. Chlorophyll is the pigment responsible for the green color in plants and plays a crucial role in the process of photosynthesis. In the presence of light, chlorophyll absorbs energy, allowing plants to convert carbon dioxide and water into glucose and oxygen.
When a leaf is kept in the light, its chlorophyll content is higher as the plant actively engages in photosynthesis. The distribution of chlorophyll in the leaf is typically more uniform, allowing for efficient light absorption and energy conversion.
In contrast, a leaf kept in the dark experiences a decrease in chlorophyll content due to the absence of light. Without light, the plant cannot undergo photosynthesis, leading to a reduced need for chlorophyll. As a result, the pattern of chlorophyll in the darkened leaf becomes less uniform, and the leaf may lose its vibrant green color, becoming pale or even yellow over time. This process is known as etiolation.
To know more about chlorophyll visit:
https://brainly.com/question/31951083
#SPJ11
Which statements are correct regarding the Law of Conservation of Matter and Energy?
Matter or energy can create itself.
Matter or energy was created by known principles of physics and chemistry.
Matter or energy can change from one form to the other.
The law agrees with the Biblical account of Creation
(more than one answer)
Answers
Answer:
Matter or energy can change from one form to the other
Explanation:
The law of conservation of energy states that energy can neither be created nor destroyed but can only be transformed i.e. changed from one form to another. For example, mechanical energy can be changed to electrical energy.
Likewise, the law of conservation of mass/matter states that matter can not be destroyed or created but can change via physical or chemical means to conserve it. For example, matter can change from liquid state to gaseous state.
From the above two laws, it can be said that "matter or energy can change from one form to the other".
in chemical cold packs, solid ammonium chloride dissolves in water forming aqueous ammonium and chloride ions. As a result of this solvation reaction, the pack feels cold on your injured ankle. write the chemical fromula incuding reactants and products. Also add energy in one of the reactant or product sides.
Answers
The chemical formula for the solvation reaction in cold packs is given below as follows:
NH₄Cl (s) + H₂O (l) ⟶ NH₄⁺ (aq) + Cl⁻ (aq)The dissolution of NH₄Cl in water is an endothermic process, and energy in the form of heat would be added to the product side of the reaction.
What is the chemical formula for solvation reaction in cold packs?The chemical formula for the solvation reaction in cold packs involving solid ammonium chloride (NH₄Cl) dissolving in water (H₂O) is given below as follows:
NH₄Cl (s) + H₂O (l) ⟶ NH₄⁺ (aq) + Cl⁻ (aq)
In this reaction, solid ammonium chloride (NH₄Cl) reacts with water (H₂O) to form aqueous ammonium ions (NH₄⁺ ) and chloride ions (Cl-). The dissolution of NH₄Cl in water is an endothermic process, meaning that energy is absorbed in the form of heat from the surroundings usually the body.
Therefore, energy in the form of heat would be added to the product side of the reaction.
Learn more about cold packs at: https://brainly.com/question/7745193
#SPJ1
How many moles do you have if you have 144 L of a gas at SATP?

Answers
Answer
moles = 5.81 mol
Explanation
Given:
Volume = 144 L
AT SATP
1 mole = 24.4651 L
Solution:
1 mole = 24.4651 L
x mole = 144 L
x = 144/24.4651
x = 5.8 mol
What a bat hits a ball what is the impulse
Answers
Answer:
Plugging in the numbers we find the average force to be Favg=18,436 N, which is equivalent to 4124 lbs of force. The impulse delivered by this force is the product of the average force the the contact time, resulting in an impulse of 12.91 Ns.
Answer:
the force multiplied by the time the objects are in contact
Explanation:
took the quiz
A new material was produced in a laboratory by bonding atoms of
different elements together in a unique arrangement. Other laboratories
were able to reproduce these results, producing the same material with
the same elements in the same arrangement. What type of matter was
produced?
*
Answers
Answer:
A compound
Explanation:
A compound is a substance formed from the chemical combination of atoms of two or more elements together.
A chemical compound always contains the same kinds of atoms of elements combined together in as specific ratio by mass and can be represented by a chemical formula. For example, water is a compound formed from the chemical combination of hydrogen and oxygen atoms in the ratio of 2 : 1. It has a chemical formula H2O.
A pure compound also always have the same chemical properties irrespective of the source of the compound. For example, chemically pure has similar properties irrespective of whether it is obtained from rain, streams or underground wells.
Therefore, if a new material was produced in a laboratory by bonding atoms of different elements together in a unique arrangement and other laboratories were able to reproduce these results, producing the same material with the same elements in the same arrangement, then the substance must be a compound.
How much energy is required to take a 22. 0-g sample of liquid water at 25. 0 °c to steam at 145. 0 °c?.
Answers
Energy required to take a 22. 0-g sample of liquid water at 25. 0 °c to steam at 145. 0 °c is 58.6 KJ.
q1 = heat required to warm the water from 25.0 °C to 100.0 °C.
= mCΔT
= 22.0 g x 4.184 J∘C−1g−1 x 75
= 6903.6 J
q2 = heat required to vapourize the water to steam at 100 °C.
= mΔH vap
= (22.0 g x 40.7 103 J/ mol )/18 g/ mol
= 49744. 4 J
q3= heat required to warm steam from 100°C to 145.0 °C.
= mCΔT
= 22.0 g x 2.01 J∘C−1g−1 x 45°C
= 1989.9 J
Total energy required to take a 22.0 g sample of liquid water at 25.0 degrees Celsius to steam at 145.0 degrees Celsius is:
= q1 + q2 + q3
= 6903.6 J + 49744. 4 J + 1989.9 J
= 58,637.9 J
= 58.6379 KJ
= 58.6 KJ
Energy is described as having the "ability to conduct work, which is the capacity to apply a force that causes an item to be displaced.
learn more about energy here
https://brainly.com/question/5144470
#SPJ4
What is te commond that alcws moung a fle from one rlase to ancherr?
Answers
The command that allows moving a file from one location to another is the "mv command".
The mv command renames or transfers files and folders from one directory to another. A file or directory keeps its base file name when moved to a new directory. All links to other files are preserved when you transfer a file, with the exception of when you move it to a different file system. A directory and its contents are added beneath the existing directory when you transfer a directory into it.
The TargetDirectory option of the mv command allows you to provide a new file name or a new directory path name when renaming a file or directory.
To knwo more about mv command
https://brainly.com/question/30737863
#SPJ11
the 3 factors that affect the process of Natural Selection
Answers
Answer:
Overproduction of Offspring. The first of these factors that must be present in order for Natural Selection to occur is the ability of a population to overproduce offspring.
Variation.
Selection.
Reproduction of Adaptations.
Explanation:
Pls help
#8: In at least three sentences, describe how the chocolate/wax is like the tectonic plates
on the surface of the Earth. Use the following terms in your answer: tectonic plates,
convection currents, mantle and density. 3 pts
Answers
Explanation:
The chocolate or wax in the activity can be compared to the tectonic plates on the surface of the Earth. Just like the tectonic plates, the chocolate or wax is made up of different layers that move and interact with each other. The movement of the tectonic plates is driven by convection currents in the mantle, which is the layer of the Earth beneath the crust. These convection currents are caused by differences in density between the hot, less dense material near the core of the Earth and the cooler, more dense material near the surface. Similarly, the movement of the chocolate or wax is driven by the heat from the flame, which causes the less dense, melted chocolate or wax to rise and the more dense, solid chocolate or wax to sink.
How many electrons are in an Fe2+ ion?
Answers

what is the character of the vapor after each condensation-vaporization cycle in a fractional distillation of a mixture?
Answers
1.) Describe the two diagrams of a bottled carbonated beverage shown below as greater
pressure or lower pressure, and then as greater solubility or lower solubility. How do these two
examples illustrate the relationship between the solubility of a gas and its vapor pressure? Explain
2.) On the diagrams below, assume that each beaker contains an equal number of moles
of solute. Label each solution as concentrated or dilute. Then indicate the approximate
relative volumes of each solution by describing the surface level on each beaker.
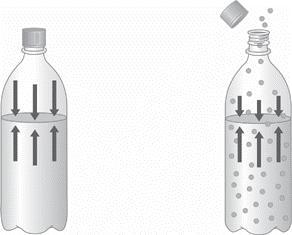

Answers
Answer:
SEE EXPLANATION
Explanation:
If i refer to the bottle at the left hand side as A and the bottle at the right hand side as B. I can say that the bottle A contains solutes which are more soluble than the solutes in bottle B. consequently, A has a lower vapour pressure than B.
We can also see that the pressure in A is much higher than in B(as indicated by the length of the arrows). The higher the pressure, the greater the solubility of the gas. Henry's Law states that: The solubility of a gas in a liquid is directly proportional to the pressure of that gas above the surface of the solution.
Again let me call the beaker on the left A and the beaker on the right B. We can see that the beaker on the left contains 10 ml of solution while the beaker on the right contains 50 ml of solution. It follows that beaker A contains a more concentrated solution since that much amount of solute is contained in a smaller volume than in beaker B as shown in the image.
11. An alloy contains 62 % by mass of aluminum and 38% by mass of unknown element .If 10.0
grams of this alloy has a volume 4.20 cm³ ,use the table of density below to identify the
unknown element in the alloy.
Element
Density g/cm³
(A) Beryllium
Copper
8.96
Aluminum
2.70
(B) Copper
Beryllium
1.85
(C) Iron
Iron
7.87
(D) Silver
Silver
10.49
Answers
Based on the given information and the densities provided in the table, the unknown element in the alloy is most likely Beryllium. option(a)
To identify the unknown element in the alloy, we need to compare the density of the alloy with the densities of the elements listed in the table.
The density of the alloy can be calculated using the given information. We know that 10.0 grams of the alloy has a volume of 4.20 cm³. Density is defined as mass divided by volume, so we can calculate the density of the alloy as:
Density = Mass / Volume = 10.0 g / 4.20 cm³ ≈ 2.38 g/cm³
Now, we compare the calculated density of the alloy (2.38 g/cm³) with the densities listed in the table. From the given options, the closest density is that of aluminum, which is 2.70 g/cm³. The alloy's density is lower than the density of aluminum, which means it must contain an element with a lower density than aluminum.
The unknown element in the alloy is most likely Beryllium (option A) with a density of 1.85 g/cm³. The combination of 62% aluminum and 38% beryllium in the alloy would result in a density close to the calculated value of 2.38 g/cm³. option(a)
For such more questions on densities
https://brainly.com/question/1749900
#SPJ8
Explain what a chemical reaction is and moles
Answers
Answer:
A process during which chemical bonds between atoms are broken and new ones are formed, producing one or more substances.
Moles are the amount of substance that contains as many particles.
Explanation:
A chemical reaction is when a chemical change occurs for example when a nail comes into contact with air and is then exposed to air it begins to rust. It forms a red-brown substance which changes the chemical composition of the original compound.
Examples of chemical reactions:
Combustion
Oxidation (rusting)
Cooking an egg
Photosynthesis
Digestion
Fireworks
Paper Burnin
The mole is the amount of substance that contains as many particles (molecules, ions or atoms) as there are in 12g of carbon.
1 mol is the amount of substance that contains the same number of particles as there are atoms in 12.0 g of carbon-12 and that number is referred to as Avogardo's constant (6.022 x \(10^{23}\)).
what happens when borax is heated strongly?
Answers
\(\\ \rm\rightarrowtail Na_2B_4H_7.10H_2O\overset{\Delta}{\longrightarrow} Na_2B_4H_7+10H_2O\)
It's very swelling\(\\ \rm\rightarrowtail Na_2B_4O_7\overset{\Delta}{\longrightarrow} NaBO_2+B_2O_3\)
number 5 plz! what ideas do you have about why there are patterns in earthquake and valcano data?

Answers
Answer:
Explanation:
The interaction along plate boundaries results in an increased frequency of earthquakes at those locations. ... Additionally, stronger earthquakes are more likely to occur along active plate boundaries. Strong earthquakes are more common at transform and convergent plate boundaries.
Calculate the number of NaBr formula units formed when 50 NBr3 molecules and 57 NaOH formula units react? 2NBr3 + 3NaOH ---> N2 + 3NaBr + 3HOBr
Answers
When 50 NBr3 molecules and 57 NaOH formula units react according to the given balanced equation, the result is the formation of 150 NaBr formula units.
According to the balanced equation provided:
2 NBr3 + 3 NaOH -> N2 + 3 NaBr + 3 HOBr
From the equation, we can see that 2 moles of NBr3 react with 3 moles of NaOH to form 3 moles of NaBr.
To determine the number of NaBr formula units formed, we need to convert the given quantities into moles.
Given:
Number of NBr3 molecules = 50
Number of NaOH formula units = 57
To convert the number of NBr3 molecules to moles, we need to divide the given quantity by Avogadro's number. Similarly, for NaOH formula units, we can directly consider them as moles.
Using Avogadro's number (6.022 x 10^23 molecules/mol), we can calculate the number of moles for NBr3 and NaOH:
Number of moles of NBr3 = 50 / (6.022 x 10^23)
Number of moles of NaOH = 57
Now, we can use the mole ratios from the balanced equation to determine the number of moles of NaBr formed. From the equation, we know that 2 moles of NBr3 react to form 3 moles of NaBr.
Number of moles of NaBr = (Number of moles of NBr3) x (3 moles of NaBr / 2 moles of NBr3)
Finally, we can convert the number of moles of NaBr to the number of NaBr formula units using Avogadro's number:
Number of NaBr formula units = (Number of moles of NaBr) x Avogadro's number
Calculating these values, we find that 50 NBr3 molecules and 57 NaOH formula units react to form 150 NaBr formula units.
To learn more about Avogadro's number click here: brainly.com/question/18948587
#SPJ11
what is the approximate ph of a 0.10 m solution of a weak acid that has a ka of 5 x 10-5 m?
Answers
2.7 is the approximate pH of a 0.10 m solution of a weak acid that has a ka of 5 x 10^-5 m.
5 x 10^-5 (0.1) = 5 x 10^-6
sqrt 5 x 10^-6 = 2.24 x 10-3
-log 2.24 x 10-3 = 2.7
pH, which originally stood for "potential of hydrogen," is a chemical scale used to quantify the acidity or basicity of an aqueous solution. Acidic solutions have a lower pH than basic or alkaline solutions. The pH of aqueous or other liquid solutions is a quantitative measure of their acidity or basicity.
The pH of a solution is a measure of its acidity or alkalinity, as well as its concentration of hydrogen ions. The pH scale often spans from 0 to 14. Aqueous solutions with a pH less than 7 at 25 ° C are acidic, whereas solutions with a pH more than 7 are basic or alkaline.
To learn more about pH, here
https://brainly.com/question/14466719
#SPJ4
Glacier striations (scrapings) were found in the deserts of Africa. What did Wegener suggest about these deserts long ago?
Answers
Wegener suggested that the deserts of Africa were once covered in ice and that the striations were formed by the movement of the ice across the desert floor.
Glacier striations are grooves or scratches in the bedrock that have been created by a glacier sliding over the surface. They are typically found in areas where the ice has melted away, revealing the bedrock beneath.
Wegener suggested that the deserts were once wetter and lusher. He proposed that the climate has gradually changed over time, making the deserts arider. This is supported by evidence of ancient riverbeds and lakes in the Sahara Desert.
Glacier striations can be used to help researchers learn about the history of a particular glacier and the climate in the area where it is located. For example, the direction of the striations can indicate the direction the glacier was moving and the depth of the striations can give clues about how deep the glacier was.
To know more about glaciers, click below:
https://brainly.com/question/6666513
#SPJ9
When the name of an anion that is part of an acid ends in -ite, what does the acid name include?
Answers
The name of an anion that is part of an acid ends in -ite, the acid name include the suffix -ous.
When an anion in chemistry has the suffix "-ite," it indicates that the anion is derived from an acid by removing one oxygen atom from the "-ate" form of the anion. The naming convention for these anions and their corresponding acids follows a specific pattern.
Let's consider the sulfate ion (SO₄²⁻). If we remove one oxygen atom from the sulfate ion, we get the sulfite ion (SO₃²⁻). The suffix "-ite" indicates that one oxygen atom has been removed.
The corresponding acid for the sulfite ion is called sulfurous acid. The prefix "sulfur-" represents the element sulfur, and the suffix "-ous" indicates that the acid is derived from the sulfite ion.
So, in general, when the name of an anion ends in "-ite," the acid name includes the prefix derived from the root name of the element, followed by the suffix "-ous."
Here are a few more examples:
Nitrate ion (NO₃⁻) becomes nitrite ion (NO₂⁻), and the corresponding acid is nitrous acid (HNO₂).
Chlorate ion (ClO₃⁻) becomes chlorite ion (ClO₂⁻), and the corresponding acid is chlorous acid (HClO₂).
Sulfate ion (SO₄²⁻) becomes sulfite ion (SO₃²⁻), and the corresponding acid is sulfurous acid (H₂SO₃).
To know more about anion here
https://brainly.com/question/14929591
#SPJ4
Determine the rate of a reaction that follows the rate law:
rate= K[A][B]", where:
k=0.2
[A] = 3 M
[B] = 3 M
m = 1
n=2
OA. 5.4 (mol/L)/s
OB. 1.2 (mol/L)/s
O C. 1.8 (mol/L)/s
OD. 27 (mol/L)/s
Answers
The rate of the reaction can be calculated using the rate law: rate = k[A][B],Therefore, the correct answer is C. 1.8 (mol/L)/s.
What is rate of the reaction ?The rate of a reaction is the speed at which a reaction occurs. It is usually expressed as the amount of reactant that is consumed or product that is produced per unit of time. The rate of a reaction depends on a number of factors including the concentration of reactants, the temperature, the presence of catalysts, and the nature of the reaction. Increasing the concentration of reactants increases the rate of the reaction, while increasing the temperature or the presence of a catalyst usually increases the rate as well.
where k is the rate constant, [A] is the concentration of A, and [B] is the concentration of B. In this case, k = 0.2, [A] = 3 M, and [B] = 3 M, so the rate of the reaction is given by: rate = 0.2 * 3 * 3 = 1.8 (mol/L)/s. Therefore, the correct answer is C. 1.8 (mol/L)/s.
To learn more about rate of the reaction
https://brainly.com/question/30706346
#SPJ1
Question 50
FCs are widely used because of their
a. Chemical stability
b. Cost
c. High toxicity
d. Atmosphere lifetime
Answers
fluorocarbons, are widely used because of their chemical stability (option a). This characteristic allows them to be utilized in various applications without breaking down easily, providing reliability and longevity.
FCs are widely used because of their chemical stability, which allows them to resist breakdown and maintain their effectiveness over time. Additionally, their cost-effectiveness makes them a popular choice for a variety of applications.
The gases known as "green house gases" are those that are thought to stop infrared rays from escaping and ultimately raise the earth's temperature.
These greenhouse gases may be synthetic or natural. The natural greenhouse gases are more challenging to regulate because they can also be created by unmanaged natural processes.
For instance, methane is formed by processes in the water and is also produced naturally through decomposition. Since this is the case, man cannot completely control the gas.
Learn more about greenhouse gases here
https://brainly.com/question/27395587
#SPJ11
What is the final temperature of a 2.50 L system when its volume is reduced to 1.75 L if the initial temperature was 298 K?
Answers
Answer:
208.6 K
Explanation:
V1/T1 = V2/T2
T2 = T1 V2 / V1
T must be in K units
T2 = 298 * 1.75 / 2.5 = 208.6 K
Homogeneous mixtures can exist
(A only In the gas phase
(B only in the solid phase
(C only in the liquid phase
(D as liquid,solid or gas
Answers
A homogeneous mixture can exist as D)Solid, liquid, or gas.
A homogeneous mixture is a collection of two or more substances that are uniform throughout their existence.
These mixtures are blended together so that the individual substance present in the mixture could not be easily distinguished or seen.
Such mixtures can exist in any state of matter. This is because a homogeneous mixture is a mixture in which the composition is uniform throughout.
If the homogeneous mixtures are seen in liquids it is called homogeneous solutions. However, homogeneous mixtures can exist only one phase at a time. More than two phases do not coexist.
Hence the correct option is D) and the answer is, Solid, liquid, or gas.
To know more about colloidal solutions, click below:
https://brainly.com/question/3003333
#SPJ9
When treated with sodium borohydride, D-glucose is converted into an alditol. (a) Draw the structure of the alditol. (b) Which L-aldohexose gives the same alditol when treated with sodium borohydride?
Answers
D-glucose changes to D-glucitol (sorbitol) when it is treated with sodium borohydride. However, L-glucose will leave the alditol (D-glucitol) stage when it is treated with sodium borohydride.
The explanation
(a) When D-glucose is treated with sodium borohydride, it is changed over into D-glucitol, additionally known as sorbitol. The structure of D-glucitol (alditol outline) is:
HOCH₂─CHOH─CHOH─CHOH─CHOH─CH₂OH
(b) The L-aldohexose that would allow the same alditol (D-glucitol) when treated with sodium borohydride is L-glucose. Since D-glucose and L-glucose are enantiomers, they have the same chemical condition but shift in their spatial course of activity.
As a result, when L-glucose is subjected to the same reaction conditions, it'll abandon the same alditol, D-glucitol, but inside the L-configuration.
Learn more about D-glucose here:
https://brainly.com/question/397060
#SPJ4
what is the colour of our oxygen
best answer will get 30 points
Answers
Answer:
colourless
Explanation:
although the gaseous form of oxygen is colourless
when it liquefies it turns into a blue fluid.
hope this helps:)
Would you expect the equivalence point ph of the wasb to be slightly acidic, neutral, or slightly basic? how does this compare to the sasb equivalence point ph?.
Answers
the WASB has a slightly acidic equivalence point and SASB has a slightly basic equivalence point. This is because WASB has a higher concentration of hydronium ions than SASB.
Hydronium ions play an important role in acid-base reactions. It is the hydrogen ion that is released or taken up in the acid-base reaction. The higher the concentration of hydronium ions, the more acidic the solution. In an acid-base reaction, an acid donates a hydrogen ion to a base. Bases accept hydrogen ions. This creates new water molecules. The equivalence point is the point at which an acid and a base react with 1:1 ratio. At this point the solution is neutral. If the acid has a higher concentration of hydronium ions than the base, the equivalence point is slightly acidic. This is due to the presence of more hydronium ions than at the start of the reaction. If the base has a higher concentration of hydronium ions than the acid, the equivalence point is slightly basic.
For more information on equivalence points, see:
Brainly.com/question/14782315
#SPJ4
Write four significance of
Scientific procedure
Answers
Answer: A question is asked and/or. A problem is identified and/or. ...
An inference is based on these observations and a. Hypothesis is made.
An experiment is designed and conducted. Results are collected and recorded.
The results are examined and. Discussed and finally. ...
SOMETIMES. Further questions are asked,
Explanation:
Example: Ba8Mu7
Beanium shroomium
Beanium shroomide
Octabeanium heptashroomide
Name these Covalent Compounds using the “Periodic Table of Food”:
7. PeMu2
8. BaPo5
9. SpMu
10. Br2E7
11. Br3Pe
12. PeE4
Answers
Answer:
Here are the answers:
7. phosphorus molybdenum diboride
8. barium phosphite
9. sulfur molybdenum
10. dibromine heptoxide
11. tribromine phosphide
12. phosphorus tetrasulfide