PLEASE HELL ME!!!!
Find the density of a cylinder on Earth that weighs 14 kg, a height of 25 cm, and a diameter of 4 cm.
Answers
Answer:
The answer is 0.045 kg/cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass of cylinder = 14 kg
Volume of a cylinder = πr²h
where
r is the radius
h is the height
\(radius = \frac{diameter}{2} \\ \)
From the question
h = 25 cm
\(r = \frac{4}{2} = 2 \: \: \: cm\)
The volume of the cylinder is
\(V = {2}^{2} (25)\pi \\ = 4 \times 25\pi \\ = 100\pi \\ = 314.159265...\)
We have
volume = 314.159 cm³
The density of the cylinder is
\(density = \frac{14}{314.159} \\ = 0.044563421...\)
We have the final answer as
0.045 kg/cm³Hope this helps you
Answer:
The answer is 0.045 kg/cm³
Explanation:
The density of a substance can be found by using the formula
From the question
mass of cylinder = 14 kg
Volume of a cylinder = πr²h
where
r is the radius
h is the height
From the question
h = 25 cm
The volume of the cylinder is
We have
volume = 314.159 cm³
The density of the cylinder is
We have the final answer as
0.045 kg/cm³
Related Questions
What is the mass of a product if the reactants have mass of 44.6 grams (Fe) and 12.8 grams (O2)?
A) 31.8 grams
B) 571 grams
C) 57.4 grams
D) 3.48 grams
Answers
Answer:
C) 57.4 grams
Explanation:
According to the law of conservation of mass/matter, mass can neither be created nor destroyed. This means that the amount of matter present in the reactants must be equal to that of the products since no amount can be lost.
Hence, according to this question which states that the mass of reactants are 44.6 grams (Fe) and 12.8 grams (O2), hence the total mass of reactants is 44.6 + 12.8 = 57.4 grams.
If the amount of mass in the reactants is 57.4 grams, then the amount of mass in the product(s) must also be 57.4 grams in conformity to the law of conservation of mass.
The mass of the product ( Fe₂O₃ ) will be ; ( C ) 57.4
The mass of the product when Fe reacts with O₂ will be equal to the summation of the masses of the reactants i.e. ∑ M₁ + M₂. and this is because of the law of conservation of mass
Reaction equation : Fe + 0₂ = Fe₂O₃
mass of Fe ( M₁ ) = 44.6 g
mass of O₂ ( M₂ ) = 12.8 g
Therefore the mass of Fe₂O₃
= 44.6 g + 12.8 g = 57.4 grams
Hence we can conclude that the mass of a product will be 57.4 grams .
Learn more : https://brainly.com/question/7041730
If a balloon with a volume of 10L at 500kpa is heated until the balloon has a temperature of 300°C, 750atm and now has a volume of 100mL. What was the original temperature of the balloon (in °C)
Answers
Answer:
103.6°C
Explanation:
We solve this problem, with the Ideal Gases Law. We know that moles of gas are not changed through time.
P . V = n . R . T
So n (number of moles) and R (Ideal Gases constant) are the same, they are cancelled. In the two different states, we can propose:
P₁ . V₁ / T₁ = P₂ . V₂ /T₂
We need to make some conversions:
100 mL . 1 L/ 1000mL = 0.1L
500 kpa . 1atm / 101.3 kPa = 4.93 atm
300°C + 273 = 573 K
We replace data:
4.93 atm . 10L / T₂ = 750 atm . 0.1L / 573K
4.93 atm . 10L = (750 atm . 0.1L / 573K) . T₂
(4.93 atm . 10L) / (750 atm . 0.1L / 573K) → T₂
T₂ = 376.65 K
We convert K to °C → 376.65 K - 273 = 103.6 °C
a.The potential for an Fe3+/Fe2+ half cell is +0.750V relative to the standard hydrogen electrode. What is its potential when using a saturated calomel electrode or a standard silver/silver chloride electrode? (Electrode potential: SCE = 0.2444V, SHE = 0.000V and Ag/Ag Cl (= 0.197V)
b. what do you understand by selective electrode?
c. Explain the various types of ion selective membrane
d. Differntiate between alternative current and direct current
e. Considering the root mean square for a sinusoidal voltage , show that
Vrms = Vpeak / 2
Answers
a. The potential for an Fe3+/Fe2+ half cell when using a saturated calomel electrode (SCE) is +0.9944V, and when using a standard hydrogen electrode (SHE) is +0.750V. When using a standard silver/silver chloride electrode (Ag/AgCl) the potential is +0.947V.
Here, correct answer will be
b. A selective electrode is an electrode that is designed to measure the activity of a specific ion in an aqueous solution. Selective electrodes are able to measure the activity of the ion of interest in the presence of other ions that are present in the solution.
c. Ion selective membranes are thin, permeable layers that allow only certain ions to pass through them. They are used in ion-selective electrodes, which are specialized electrical meters that measure the activity of a particular ion in a solution. Ion-selective membranes can be made from organic materials, such as cellulose acetate, or from inorganic materials, such as silica.
d. Alternating current (AC) is an electric current that reverses direction periodically. In contrast, direct current (DC) is an electric current that flows in one direction only.
Know more about potential here
https://brainly.com/question/4305583#
#SPJ11
which statement regarding the credentialing of a medical assistant is true? A. Both the RMA and CMA credentials are obtained through the Association of Medical Technologists.
B. CMA credentialing is obtained through the American Association of Medical Assistants (AAMA).
C. CMA-eligible students can graduate from a program accredited by the United States Department of Education.
D. RMA-eligible students must graduate from a CAAHEP or ABHES accredited academic program.
Answers
The statement which is true about credentialing of a medical assistant is that CMA credentialing is obtained through the American Association of Medical Assistants (AAMA), thus option B is correct.
The CMA credential designates a medical assistant who has achieved certification through the Certifying Board of the American Association of Medical Assistants (AAMA).
The CMA has been educated and tested in a wide scope of general, clinical, and administrative responsibilities as outlined in the Content Outline for the CMA Certification Exam.
Every day the AAMA responds to more than 100 employer requests for CMA certification verification—for both current and potential employees.
The CMA Fact Sheet offers a quick take on the reasons a CMA credential attests to medical assistants’ high level of knowledge and competence.
Thus, statement which is true about credentialing of a medical assistant is that CMA credentialing is obtained through the American Association of Medical Assistants (AAMA), thus option B is correct.
Learn more about CMA credentialing,here:
https://brainly.com/question/33724057
#SPJ12
A new sunscreen has been developed that is supposed to be more effective at preventing sunburn. 30 participants spray one arm with the new formula, and spray the other arm with the leading formula. After 4 hours in the sun, their skin is evaluated for any redness. What is the purpose of the experiment? Identify the independent variable. Identify the dependent variable. Describe the first group of people in the experiment. Describe the second group of people in the experiment. Identify two factors which are kept constant( same). Which group is the test group? Which group is used for comparison?
Answers
Answer:
Explanation:
This question appears incomplete but can however still b answered.
The purpose of the experiment is to test the effectiveness of the new sunscreen.
An independent variable is the variable that is changed (directly or indirectly) during the course of an experiment. The independent variable here are the sunscreen.
A dependent variable is the variable that is been tested in the course of the experiment. The dependent variable here is the redness of the skin.
The first group here (this could be in the missing part of the question, hence response not certain) is the control group. The control group consists of the arms sprayed with the leading/old sunscreen formula.
The second group is the test group. It consists of the arms sprayed with the new sunscreen formula.
The factors that are kept constant (or control variable) are the duration under the sun (4 hrs) and the participants' arms used.
The test group is the second group while the group used for comparison (to be sure the new sunscreen is more effective) is the first group.
The chemical equation below shows the decomposition of ammonium nitrate (NH4NO3). NH4NO3 Right arrow. N2O 2H2O A chemist who is performing this reaction starts with 160. 1 g of NH4NO3. The molar mass of NH4NO3 is 80. 03 g/mol; the molar mass of water (H2O) is 18. 01 g/mol. What mass, in grams, of H2O is produced? 9. 01 18. 01 36. 03 72. 6.
Answers
The mass of water, H₂O produced from the reaction is 72.06 g
We'll begin by calculating the mass of NH₄NO₃ that reacted and the mass of H₂O produced from the balanced equation.
NH₄NO₃ —> N₂O + 2H₂O
Molar mass of NH₄NO₃ = 80.03 g/mol
Mass of NH₄NO₃ from the balanced equation = 1 × 80.03 = 80.03 g
Molar mass of H₂O = 18.01 g/mol
Mass of H₂O from the balanced equation = 2 × 18.01 = 36.02 g
From the balanced equation above,
80.03 g of NH₄NO₃ reacted to produce 36.02 g of H₂O
Finally, we shall determine the mass of H₂O produced by the reaction of 160.1 g of NH₄NO₃.
From the balanced equation above,
80.03 g of NH₄NO₃ reacted to produce 36.02 g of H₂O.
Therefore,
160.1 g of NH₄NO₃ will react to produce = (160.1 × 36.02) / 80.03 = 72.06 g of H₂O.
Thus, the mass of water, H₂O produced from the reaction is 72.06 g
Learn more on stiochoimetry: https://brainly.com/question/15048051
metallic bonding occurs between metal atoms that have____
Answers
Positively charged metal ions.
How metal bonding works?Metallic bonding is a type of chemical bonding caused by the electrostatic attraction of conduction electrons (in the form of an electron cloud of delocalized electrons) to positively charged metal ions.
As chemistry matured into a discipline, it became obvious that metals dominated the periodic table of elements, and significant progress was made in the description of the salts that may be generated in interactions with acids. With the advent of electrochemistry, it became known that metals normally enter solution as positively charged ions, and the oxidation processes of the metals in their electrochemical series were well recognized. Metals were shown as positive ions bound together by an ocean of negative electrons.
To learn more about metal bonding to refer:
https://brainly.com/question/29762857
#SPJ4
1. Which of the following components does not make up rock salt? *

Answers
Answer:
In my opinion the third one is write.
Explanation:
soil.
it is found that 25.00 ml of the antacid solution required 13.7 ml of a naoh solution to titrate it to a methyl red end point. it takes 29.2 ml of this naoh solution to neutralize 25.00 ml of the original (before adding antacid) hcl acid. how much of the antacid solution (in ml) has been neutralized in the 25.00 ml sample that was titrated?
Answers
468 mL of the antacid solution was neutralized in the 25.00 mL sample that was titrated.
C(NaOH) = (mol NaOH) / (V(NaOH) in L)
where,
mol NaOH = mol HCl (since they react in a 1:1 ratio)
= (C(HCl) x V(HCl)) in moles
= (0.1 M x 0.025 L) in moles
= 0.0025 moles
V(NaOH) in L = 29.2 mL / 1000 mL/L
= 0.0292 L
C(NaOH) = 0.0025 moles / 0.0292 L
= 0.0856 M
calculate the amount of acid that was neutralized:
mol NaOH used in titration = C(NaOH) x V(NaOH) used in titration
= 0.0856 M x 0.0137 L
= 0.00117 moles
mol acid in 25.00 mL of antacid solution = 0.00117 moles
mol acid in 1000 mL of antacid solution = (0.00117 moles / 0.025 L) x 1000 mL/L
= 46.8 moles/L
So the volume of the antacid solution that was titrated is:
V(titrated) = (46.8 moles/L) / (0.1 moles/L)
= 468 mL
Antacids are a group of medications used to neutralize stomach acid, relieve heartburn, and treat acid indigestion. Antacid solutions are formulations that contain an active ingredient that reacts with hydrochloric acid (HCl) in the stomach to form a salt and water, thereby reducing the acidity of the stomach.
Most antacid solutions contain alkaline substances such as calcium carbonate, magnesium hydroxide, aluminum hydroxide, and sodium bicarbonate. These substances act as bases, reacting with HCl to form salt and water. Antacid solutions work by increasing the pH of the stomach, which helps to reduce symptoms such as heartburn, acid indigestion, and upset stomach. Antacids are available in various forms, including tablets, liquids, and chewable tablets.
To learn more about Antacid solution visit here:
brainly.com/question/30897207
#SPJ4
what is indicated by the shape of the titration curve? a. a diprotic acid was titrated with a strong base. b. a triprotic acid was titrated with a strong base. c. a diprotic base was titrated with a strong acid. d. a triprotic base was titrated with a strong acid.
Answers
Option C a diprotic base was titrated with a strong acid is indicated by the shape of the titration curve.
The titration curve is a plot of the analyte solution's pH versus the volume of titrant added as the titration progresses. Titrations are frequently documented on graphs known as titration curves, which generally include the quantity of the titrant also as explanatory variables and the pH value of the solution as the predictor variables. The equivalence point of the graph is where the titrant has nullified all of the starting solution. Titration curves are a low-cost, valuable, and versatile method for gaining sophisticated information about the acidity of acidic water. The information on the current buffer system's strength can aid in understanding and documenting the complicated nature of acidified mining moisture buffer systems.
Learn more about titration curve here:
https://brainly.com/question/29590776
#SPJ4
Please Research how the chemist John Dalton came up with his ideas about atoms and review how scientific ideas change with time.
Answers
Answer:
Experiments with gases that first became possible at the turn of the nineteenth century led John Dalton in 1803 to propose a modern theory of the atom based on the following assumptions. 1. Matter is made up of atoms that are indivisible and indestructible.
Given the reaction at equilibrium: 2 CO (g) + O2 (g) <---> 2 CO2 (g)
When the reaction is subjected to pressure, the equilibrium will shift to the ______.
Select one:
a. the pressure can't be changed
b. no change will occur
c. left
d. right
Answers
When the reaction is subjected to pressure, the equilibrium will shift to the side with fewer gas molecules, which is the left side. Therefore, the answer is c. left.
According to Le Chatelier's principle, when a system at equilibrium is subjected to a change in pressure, it will respond by shifting in a way that reduces the effect of the change. In this case, increasing the pressure would cause the equilibrium to shift towards the side with fewer gas molecules to alleviate the increase in pressure.
Since there are fewer gas molecules on the left side of the reaction (2 CO + O2), the equilibrium will shift to the left to reduce the total number of gas molecules. This means that the concentrations of CO and O2 will increase, while the concentration of CO2 will decrease until a new equilibrium is established. Thus, the equilibrium will shift to the left.
To learn more about gas molecules click here
brainly.com/question/30832032
#SPJ11
A cylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon, and 5.00 moles of helium.
If the gas mixture is at STP, what is the partial pressure of the argon?
Answers
PRACTICE MULTIPLE CHOICE QUESTIONS FROM UNIT 10
1.) A cylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon, and 5.00 moles of helium. If
the gas mixture is at STP, what is the partial pressure of the argon?
(A) 152 torr (B) 228 torr (C) 380. torr (D) 760. torr
2.) Compared to the average kinetic energy of 1 mole of water at 0 oC, the average kinetic energy
of 1 mole of water at 298 K is
(A) the same, and the number of molecules is the same
(B) the same, but the number of molecules is greater
(C) greater, and the number of molecules is greater
(D) greater, but the number of molecules is the same
3.) If the pressure on a given mass of gas in a closed system is increased and the temperature
remains constant, the volume of the gas will
(A) decrease (B) increase (C) remain the same
4.) Which gas has approximately the same density as C2H6 at STP?
(A) NO (B) NH3 (C) H2S (D) SO2
5.) At a temperature of 273 K, a 400. milliliter gas sample has a pressure of 760. millimeters of
mercury. If the pressure is changed to 380. millimeters of mercury, at which temperature will
this gas sample have a volume of 551 milliliters?
(A) 100 K (B) 188 K (C) 273 K (D) 546 K
Get the answers to these questions
Back to the Unit 10 Old Tests Page
Back to the Unit 10 Page
Back to the Main Page
Considering the Dalton's partial pressure and STP conditions, the total pressure of argon in the mixture of gases is 0.3 atm.
On one side, the STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C are used and are reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
On the other side, the pressure exerted by a particular gas in a mixture is known as its partial pressure.
So, Dalton's law states that the total pressure of a gas mixture is equal to the sum of the pressures that each gas would exert if it were alone:
\(P_{T} =P_{1} +P_{2} + ... + P_{n}\)
where n is the number of gases in the gaseous sample.
Dalton's partial pressure law can also be expressed in terms of the mole fraction of the gas in the mixture.
So in a mixture of two or more gases, the partial pressure of gas A can be expressed as:
\(P_{A} =x_{A} P_{T}\)
In this case, the mole fraction of the argon can be calculated knowing that:
number of moles of argon= 3 molestotal number of moles= 2 moles of nitrogen + 3 moles of argon + 5 moles of helium= 10 molesThen:
\(x_{argon} =\frac{3 moles}{10 moles} = 0.3\)
So, if the gas mixture is at STP, the total pressure of the gas mixture is 1 atm and the partial pressure of argon can be calculated as:
\(P_{argon} =x_{argon} P_{T}\)
\(P_{argon} =0.3x 1 atm\)
\(P_{argon} =0.3 atm\)
In summary, the total pressure of argon in the mixture of gases is 0.3 atm.
Learn more:
brainly.com/question/14239096?referrer=searchResults brainly.com/question/25181467?referrer=searchResults brainly.com/question/14119417Please help me due in like 2 min
A model of the water cycle was made by adding a small amount of water into an aquarium, covering the aquarium with a piece of glass, and placing a lamp over the glass cover to add heat. What does the lamp represent when comparing this model to the real-life water cycle?
Clouds
Precipitation
The ocean
The sun
Answers
Answer: D) The sun
Explanation:
Help Me Please
The fuel used to power the booster rockets on space shuttles is a mixture of aluminum metal and ammonium perchlorate. The following balanced equation represents the reaction.
3Al + 3NH₄ClO₄ → Al₂O₃ + AlCl₃ + 3NO + 6H₂O
What is the mole ratio of Al to Al₂O₃?
Al:Al₂O₃ = 3:___.

Answers
The mole ratio of the Al:Al₂O₃ is given from the question that we have as 3:1
What is mole ratio?A mole ratio is the ratio of the number of moles of one substance to the number of moles of another substance in a chemical reaction. It is often used to determine the stoichiometry of a reaction, which is the relationship between the relative amounts of reactants and products.
Mole ratios can be used to determine the amounts of reactants and products needed to achieve a specific reaction yield, or to calculate the expected yield of a reaction.
Learn more about mole ratio:https://brainly.com/question/15288923
#SPJ1
For the reaction of aluminum metal and ammonium perchlorate, the mole ratio of Al to Al₂O₃ is 3 : 1 (3 is to 1).
How to find the mole ratio?Mole ratio requires a balanced equation, the coefficients of the elements and a division of the coefficients.
To find the mole ratio of Al to Al₂O₃, we need to look at the coefficients in the balanced equation.
The balanced equation is:
3Al + 3NH₄ClO₄ → Al₂O₃ + AlCl₃ + 3NO + 6H₂O
The coefficient of Al is 3 and the coefficient of Al₂O₃ is 1.
Therefore, the mole ratio of Al to Al2O3 is 3:1.
Learn more on mole ratios here: https://brainly.com/question/26023
#SPJ1
Aluminum is more reactive than carbon.Use this fact to explain why tin has been used by humans for longer than pure aluminium.
Answers
Answer:
aluminium is more reactive than carbon. carbon is used to displace less reactive metals from their compounds. this happens since the carbon reacts with the other substance(s) in the compound, and the less reactive metal does not.
Answer:
Explanation: aluminium is more reactive than carbon. carbon is used to displace less reactive metals from their compounds. this happens since the carbon reacts with the other substance(s) in the compound, and the less reactive metal does not.It is highly reactive, though the metal is protected by a surface layer of inert transparent oxide (Al2O3) that forms rapidly in air, providing excellent corrosion resistance.
Help would be greatly appreciated, thank you!! :) What is the relationship between Pressure (P) and Volume (V)?
1. Inversely proportional
2. Directly proportional
3. There is no relationship
Answers
Which of the following is a disadvantage of increasing our use of solar energy?It would reduce our dependence on fossil fuels.Energy from the sun is abundant.Currently, the production and storage of solar energy is bit as cost efficient when compared to obtaining the same quantity of energy from fossil fuels.Solar energy does not generate air pollution such as nitrogen oxides and sulfur oxides.
Answers
The disadvantage of increasing our use of solar energy is that unfortunately currently, the production and storage of solar energy is bit as cost efficient when compared to obtaining the same quantity of energy from fossil fuels.
How many grams are in 9.05 x 1023 atoms of silicon
Answers
Answer:
42.2075 grams
Explanation:
gentian violet is a dye using in dna gel electrophoresis it is yellow in strongly acidic solutions and purple in solutions ______
Answers
Gentian violet, a dye used in DNA gel electrophoresis, exhibits a yellow color in strongly acidic solutions and turns purple in solutions with higher pH levels, such as neutral or basic solutions. This color change aids in the visualization of DNA fragments during the gel electrophoresis process.
Gentian violet is a common dye used in DNA gel electrophoresis to stain DNA bands. It is a cationic dye that binds to DNA molecules, making them visible under UV light. Gentian violet appears yellow in strongly acidic solutions and purple in solutions with a higher pH. During electrophoresis, the DNA is separated by size and charge, resulting in distinct bands on the gel. Gentian violet stains these bands, allowing scientists to visualize the DNA fragments. However, excessive use of gentian violet can damage DNA, so it is important to use it in moderation. In summary, gentian violet is a vital tool for DNA analysis, but its use must be carefully controlled to prevent any negative effects on the DNA samples.
To know more about gel electrophoresis visit:
https://brainly.com/question/30791630
#SPJ11
Complete and balance the equation for the thermal decompositon of potassium chlorate.
If 9.50 moles of oxygen is produced, how much heat is also produced? The heat of reaction is -89.4 kJ.
If you start with 307 grams of potassium chlorate, how many liters of oxygen will be produced at 723 torr and 32.0 °C?.
How much heat is produed when 307 grams of potassium chlorate is decomposed?
If the heat from the reaction was all absorbed by the 74.2 liter of collection water at 14.3 °C, what would the final temperature of the collection water?
Answers
Answer:
1. The balanced equation for the thermal decomposition of potassium chlorate is:
2KClO3(s) → 2KCl(s) + 3O2(g)
2. Using the stoichiometry of the balanced equation, we can see that 2 moles of KClO3 produce 3 moles of O2. Therefore, 9.50 moles of O2 are produced by (9.50 moles O2 / 3 moles O2 per 2 moles KClO3) = 6.33 moles KClO3. The heat produced by the decomposition of 6.33 moles of KClO3 is:
q = nΔHrxn = (6.33 mol)(-89.4 kJ/mol) = -566 kJ
3. To solve this problem, we need to use the ideal gas law to calculate the volume of O2 produced. First, we convert 307 g of KClO3 to moles:
n = m/M = 307 g / 122.55 g/mol = 2.50 mol KClO3
Using the stoichiometry of the balanced equation, we can see that 2 moles of KClO3 produce 3 moles of O2. Therefore, 2.50 moles of KClO3 produce (3/2 x 2.50) = 3.75 moles of O2. Now we can use the ideal gas law to calculate the volume of O2 produced:
PV = nRT
V = nRT/P = (3.75 mol)(0.08206 L·atm/mol·K)(305.15 K)/(723 torr/760 torr/atm) = 8.59 L
4. The heat produced by the decomposition of 307 g of KClO3 is:
n = m/M = 307 g / 122.55 g/mol = 2.50 mol KClO3
q = nΔHrxn = (2.50 mol)(-89.4 kJ/mol) = -223 kJ
5. We can use the equation q = mcΔT to calculate the final temperature of the water. First, we need to calculate the heat capacity of the water:
C = mc = (74.2 L)(1.00 kg/L)(4.18 J/g·K) = 310 kJ/K
Now
Explanation:
Who turned the ragged Continental Army into a more efficient fighting force?
Answers
Calculate the molarity of solution of "sodium sulfate" that contains 5. 2 grams sodiums sulfate diluted to 500mL
Answers
The molarity of the sodium sulfate solution is 0.0732 M.
To calculate the molarity of a sodium sulfate solution that contains 5.2 grams of sodium sulfate diluted to 500 mL, we need to convert the mass of sodium sulfate to moles and divide it by the volume in liters.
First, we calculate the molar mass of sodium sulfate:
Na = 22.99 g/mol (atomic mass of sodium)
S = 32.07 g/mol (atomic mass of sulfur)
O = 16.00 g/mol (atomic mass of oxygen)
Molar mass of Na2SO4 = (2 * 22.99) + 32.07 + (4 * 16.00) = 142.04 g/mol
Next, we convert the mass of sodium sulfate to moles:
moles = mass / molar mass
moles = 5.2 g / 142.04 g/mol = 0.0366 mol
Now, we convert the volume of the solution to liters:
volume (in liters) = 500 mL / 1000 mL/L = 0.5 L
Finally, we calculate the molarity of the solution:
molarity (M) = moles / volume
molarity (M) = 0.0366 mol / 0.5 L = 0.0732 M
Therefore, the molarity of the sodium sulfate solution is 0.0732 M.
Learn more about molarity here:
https://brainly.com/question/31545539
#SPJ11
What is the speed of a jet plane that flies 78500 km in 10.5 hours (in km/hr)?
Do not use decimal
Answers
S = 78500/10.5
S = 7,476 Knots
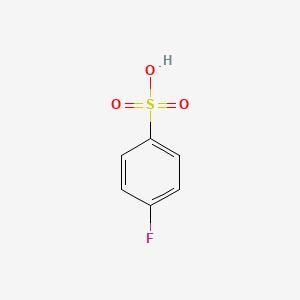
p‑fluoroanisole reacts with sulfur trioxide and sulfuric acid. draw the major product of this substitution reaction; if applicable, minimize formal charges via expanded octets. assume 1 equivalent of reagents is used.
Answers
The major product of the reaction between p‑fluoroanisole and sulfur trioxide and sulfuric acid is p‑fluorobenzene sulfonic acid. The sulfonic acid group (-SO3H) substitutes for the methoxy group (-OCH3) on the benzene ring. The product is shown below:
F
|
H3C--O--C6H4--SO3H
|
H
Note that the sulfur atom has an expanded octet, with 10 electrons in its valence shell.
One of the most well-known instances of an electrophilic aromatic substitution reaction is the reaction of p-fluoroanisole with sulphur trioxide and sulfuric acid. The electrophile in this reaction is a highly reactive sulphur trioxide-sulfuric acid complex, and the sulphur trioxide and sulfuric acid serve as its sources. The electrophile assaults the p-electron-rich fluoroanisole's aromatic ring, removing the methoxy group and replacing it with a sulfonic acid (-SO3H) group.
P-fluorobenzenesulfonic acid is a helpful intermediate in many chemical synthesis reactions due to the sulfonic acid group's high acidity and polarity. Additionally, a variety of chemical processes, including esterification, amidation, and reduction, can be used to further modify the sulfonic acid group in order to produce other derivatives.
The sulfonic acid group's sulphur atom has an extended octet, which implies it contains more than eight valence electrons in its outer shell. For lesser elements like carbon, nitrogen, and oxygen, this is unusual, but for heavier elements like sulphur and phosphorus, it is rather typical. Expanded octets are typically seen when the central atom can interact with empty d orbitals that can form bonds.
For more question on substitution reaction click on
https://brainly.com/question/27906533
#SPJ4
How many elements are in this chemical formula?
2(NH4)2SO4
Your answer

Answers
Answer:
4
Explanation:
Nitrogen (N)
hydrogen (H)
sulfur (S)
oxygen (O)
Animals caught as bycatch usually suffocate and are thrown back into the sea. Do you feel that the harm
caused from bycatch is worth the benefit of having reasonably priced seafood in grocery stores? Explain
Answers
Bycatch can negatively affect species consisting of seafood dolphins, sea turtles, protected fish, and whales by way of harming animals, contributing to population declines, and impeding populace recuperation.
Bycatch occurs because modern fishing is very efficient, often covers an extensive area, and can be highly unselective different influences of fisheries on marine mammals may additionally encompass elimination of their favored prey and once in a while habitat harm.
Bycatch is part of most business fishing operations. it's miles created whilst marine life is by chance stuck with the aid of fishing vessels. Fish and other undesirable aquatic animals are then tossed and returned to the ocean. Many are both lifeless or lose of life when they're thrown lower back.
Despite new technologies and the enterprise's reputation the difficulty, of bycatch continues to be a first-rate problem. now not handiest does it cause avoidable deaths and injuries, but the fishing strategies can be dangerous to the marine environments wherein they're employed.
Learn more about Bycatch here:-https://brainly.com/question/15574285
#SPJ9
Ned help with this question

Answers
Answer:
135.6
Explanation:
12 * 11.3 = 135.6
To find the mass you have to multiply the density and volume together
If you already have the mass you divide the mass by either the density or volume
If 24k gold is the highest purity (100%), then a 6 Karat piece of gold jewelry would contain what percentage of gold?
Answers
Answer:
25% gold.
Explanation:
6 is 25 percent of 24, so 6 karat gold would be 25% gold.
Question 9 (Essay Worth 4 points)
(01.01 MC)
Read the two questions.
Question 1: Is change in atmospheric CO₂ levels likely to impact marine life?
Question 2: Should diesel and gasoline engines compliant with CO₂ emission standards be banned?
Use complete sentences to explain whether both questions can be answered by science or not. Be sure to explain why for each question.
Answers
Changes in the level of atmospheric CO₂ will affect marine organisms such as crustaceans.
Diesel and gasoline engines compliant with CO₂ emission standards not be banned.
What is the effect of atmospheric CO₂ levels likely to impact marine life?Atmospheric CO₂ refers to carbon dioxide present in the atmosphere.
Atmospheric CO₂ is required by marine organisms such as crustaceans to make their shells.
Therefore, changes in the level of atmospheric CO₂ will affect the marine organisms such that depend on it to make its shells such as crabs.
Diesel and gasoline engines are associated with CO₂ emission. However, an increase in CO₂ emission has been implicated in global warming as CO₂ is a known greenhouse gas.
Therefore, diesel and gasoline engines compliant with CO₂ emission standards not be banned.
In conclusion, increase in atmospheric CO₂ levels due to increased emission leads to global warming.
Learn more about CO₂ emission at: https://brainly.com/question/12484795
#SPJ1