Part C Before you begin, keep in mind these two points: The timer runs fast, so the minutes go faster than actual minutes. The temperature will rise during the experiment. If the temperature gets very high, lower it to around 300 K. Follow these steps, and then record your observations: Locate the orange reset button on the bottom right side of the screen. Press reset to start the reaction over. Drag the top of the ruler upward until it reaches the 40 mark. Drag the left platform upward until the top of the platform coincides with the 30 mark on the ruler. Toggle the blue play/pause button to the play position at the bottom of the screen to ensure that the reaction doesn’t start before you’re ready. Add 50 A particles, and press the play button on the bottom. Immediately start the timer using the play button on the blue box. At every minute on the timer, pause the simulation and record the number of A and B particles.

Answers
The given instructions outline the steps to follow in a simulation or experiment involving particles A and B. The purpose is to observe and record the number of A and B particles at each minute on the timer. The exact observations and data collection will depend on the specific simulation or experiment being conducted.
Locate the orange reset button: Find the reset button on the bottom right side of the screen and press it to start the reaction over. This ensures that the previous data is cleared and the experiment begins from the initial state.
Drag the ruler: Use the mouse or touch screen to drag the top of the ruler upward until it reaches the 40 mark. This step sets the reference point for measuring the positions of the particles.
Adjust the left platform: Drag the left platform upward until the top of the platform aligns with the 30 mark on the ruler. This step positions the platform for the particles to interact within the desired range.
Toggle the blue play/pause button: Locate the blue play/pause button at the bottom of the screen. Make sure it is in the play position to prevent the reaction from starting before you are ready. This allows you to control the timing of the experiment.
Add 50 A particles: Use the appropriate tool or feature to add 50 A particles to the simulation or experimental setup. This step ensures that the initial condition includes a specific number of A particles.
Start the timer and record observations: Press the play button on the blue box to start the simulation or experiment. Immediately start the timer using the play button on the timer itself. At every minute on the timer, pause the simulation and record the number of A and B particles. This step allows you to track the changes in the particle population over time.
Note: The specific details and actions may vary depending on the simulation or experiment being conducted. It is important to follow the given instructions accurately and record the observations as instructed.
For more such questions on experiment , click on:
https://brainly.com/question/26117248
#SPJ8
Related Questions
Suppose 4.0 g of hydrogen reacts completely with 32.0 g of oxygen to form one product what is the mass of the product?
Answers
Answer: The mass of product, \(H_2O\) is, 36.0 grams.
Explanation : Given,
Mass of \(H_2\) = 4.0 g
Mass of \(O_2\) = 32.0 g
Molar mass of \(H_2\) = 2 g/mol
Molar mass of \(O_2\) = 32 g/mol
First we have to calculate the moles of \(H_2\) and \(O_2\).
\(\text{Moles of }H_2=\frac{\text{Given mass }H_2}{\text{Molar mass }H_2}\)
\(\text{Moles of }H_2=\frac{4.0g}{2g/mol}=2.0mol\)
and,
\(\text{Moles of }O_2=\frac{\text{Given mass }O_2}{\text{Molar mass }O_2}\)
\(\text{Moles of }O_2=\frac{32.0g}{32g/mol}=1.0mol\)
Now we have to calculate the limiting and excess reagent.
The balanced chemical equation is:
\(2H_2+O_2\rightarrow 2H_2O\)
From the balanced reaction we conclude that
2 mole of \(H_2\) react with 1 mole of \(O_2\)
From this we conclude that, there is no limiting and excess reagent.
Now we have to calculate the moles of \(H_2O\)
From the reaction, we conclude that
2 moles of \(H_2\) react to give 2 moles of \(H_2O\)
Now we have to calculate the mass of \(H_2O\)
\(\text{ Mass of }H_2O=\text{ Moles of }H_2O\times \text{ Molar mass of }H_2O\)
Molar mass of \(H_2O\) = 18 g/mole
\(\text{ Mass of }H_2O=(2.0moles)\times (18g/mole)=36.0g\)
Therefore, the mass of product, \(H_2O\) is, 36.0 grams.
Determine the pressure, in atm, for 3.66 Liters of NH3 gas at a temperature of 298 K and a
mass of 10.0 g?
Answers
Answer:
3.92 atm
Explanation:
The formula for the pressure of a gas is P = nRT/V. We have the following:
R - 0.0821 (gas constant)
T - 298 K
V - 3.66 L
n - ? (in moles)
We have to find the moles of the NH₃ gas. To do that, we have to divide the mass of the NH₃ gas by the molar mass of NH₃.
10.0 g × 1 mol NH₃/17.03 g NH₃ = 0.587 moles (rounded to 3 decimal places)
Now we have all the information needed to solve for the pressure.
P = nRT/V
P = (0.587)(0.0821)(298) ÷ 3.66
P = 14.3614246 ÷ 3.66
P = 3.923886503 ⇒ 3.92 atm (rounded to 3 decimal places)
The pressure of the NH₃ gas at a temperature of 298 K and a mass of 10.0 g is 3.92 atm.
Hope that helps.
4.0 g Mg and 4.0 g O2 are placed in a container and magnesium oxide, MgO, forms. The Mg is totally consumed but 1.4 g O2 remains. How much magnesium oxide formed
Answers
Answer:
The correct answer is: 6.6 g MgO
Explanation:
First we have to write and balance the chemical reaction as follows:
2Mg(s) + O₂(g) → 2MgO(s)
That means that 2 moles of Mg(s) react with 1 mol of O₂(g) to give 2 moles of MgO(s). If Mg is totally consumed and a mass of O₂ remains unaltered after reaction, the limiting reactant is Mg. We use the limiting reactant to calculate the mass of product.
According to the balanced chemical equation, 2 moles of Mg(s) produce 2 moles of MgO(s).
2 moles Mg = 2 mol x molar mas Mg= 2 mol x 24.3 g/mol = 48.6 g Mg
2 moles MgO= 2 mol x (molar mass Mg + molar mass O) = 2 mol x (24.3 g/mol + 16 g/mol) = 80.6 g MgO
The stoichiometric ratio is 80.6 g MgO/48.6 g Mg. So, we multiply this ratio by the mass of consumed Mg (4.0 g) in order to obtain the produced mass of MgO:
4.0 g Mg x 80.6 g MgO/48.6 g Mg = 6.63 g MgO
6.6 grams of magnesium oxide are formed.
An equilibrium mixture of N2, 02, and NO gases at 1500 K is determined to consist of
6.4 x101-3 mol/1 oF N2, 1.7 x 101-3 mol/ of 02 , and 1.1 × 10 ^-5 mol/1 of NO. What is the equilibrium constant for the system at this temperature?
Answers
The equilibrium constant for the system at this temperature is\(1.17 × 10^-31 mol^2/L^2\).
For the chemical equation:
N2(g) + O2(g) ⇌ 2NO(g)
The equilibrium mixture at a temperature of 1500 K is determined to contain 6.4 × 10^-3 mol/L of N2,\(1.7 × 10^-3\)mol/L of O2 and 1.1 × 10^-5 mol/L of NO. First, we need to calculate the concentration of N2 and O2 required to produce
1.1 × 10^-5 mol/L of NO:
2NO(g) = N2(g) + O2(g)
Given that there are 1.1 × 10^-5 mol/L of NO, the number of moles of N2 and O2 are equal since the stoichiometric ratio is 1:1. Therefore:
\(1.1 × 10^-5 mol/L\) = [N2][O2]Kc = \(([NO]^2)/([N2][O2])Kc\)= \((1.1 × 10^-5 mol/L)^2/(6.4 × 10^-3 mol/L)(1.7 × 10^-3 mol/L)Kc\) =
1.17 × 10^-31 mol^2/L^2.
for such more questions on equilibrium
https://brainly.com/question/5081082
#SPJ8
5. Infer if a water molecule (H20) has two hydrogen atoms and one oxygen atom, how would you describe the make- up of a carbon dioxide molecule (CO2)?
(please help)

Answers
Answer:
The correct answer is - one carbon atom and two oxygen atoms.
Explanation:
Carbon dioxide is a gas that is present in the atmosphere of the earth. The chemical formula of carbon dioxide is CO₂. In this colorless gas, there is one molecule of carbon dioxide is made up of one atom of carbon covalently double bonded to the two atoms of the oxygens.
Thus, the make- up of a carbon dioxide molecule (CO2) includes one carbon atom and two oxygen atoms.
Given the reaction below, which is the being reduced?
Mg + Cl2 Right arrow. Mg2+ + 2Cl–
2CI–
CI2
Mg
Mg2+
Answers
In the given reaction, chlorine (\(Cl_{2}\)) is being reduced.
In the given reaction:
Mg + \(Cl_{2}\)→ \(Mg^2^+\) + 2\(Cl^-\)
The reactants are magnesium (Mg) and chlorine gas ( \(Cl_{2}\)), and the products are magnesium cations (\(Mg^2^+\)) and chloride anions (\(Cl^-\)).
To determine which species is being reduced, we need to compare the oxidation states (or oxidation numbers) of the elements before and after the reaction. The element that undergoes a decrease in oxidation state is being reduced.
In this reaction, the oxidation state of magnesium changes from 0 to +2. Since the oxidation state of magnesium increases, it is undergoing oxidation, not reduction.
On the other hand, the oxidation state of chlorine changes from 0 to -1. The chlorine atoms in \(Cl_{2}\) have an oxidation state of 0, while in the chloride ions (\(Cl^-\)), the oxidation state is -1. Since the oxidation state of chlorine decreases, it is being reduced.
Therefore, in the given reaction, chlorine ( \(Cl_{2}\)) is being reduced. It gains electrons and undergoes a decrease in oxidation state from 0 to -1.
for more questions on reduced
https://brainly.com/question/14854495
#SPJ8
During the titration, a student pulls out the pH electrode from the titration beaker several times (with about 0.25 mL of solution on it each time) and rinses it off with DI water into a waste container. Will this affect the measured equivalent mass? If so, will the equivalent mass come out higher or lower?
Answers
The student pulling out the pH electrode from the titration beaker and rinsing it off with DI water into a waste container several times during the titration will not significantly affect the measured equivalent mass.
This is because the equivalent mass of a substance is determined by the stoichiometry of the reaction, which is not influenced by the pH electrode or the rinsing process. However, it is important to note that if the student is rinsing the electrode with a significant amount of water, it could dilute the solution and affect the accuracy of the titration. Therefore, it is recommended to use a minimal amount of water during the rinsing process to minimize any potential dilution effect.
To know more about titration, here
brainly.com/question/31271061
#SPJ1
Why does the solubility of alkaline earth metal hydroxides in water increase down the group?
Answers
Answer:
The solubility of alkaline earth metal hydroxides in water increases down the group because the size of the metal cation increases as you move down the group. This increase in size results in a decrease in the cation's charge density, which makes it less able to attract and hold onto hydroxide ions. As a result, the hydroxides become more soluble in water as you move down the group. Additionally, the lattice energies of the hydroxides decrease down the group, making it easier to break apart the crystal lattice structure and dissolve the hydroxides in water.
a student performs a gravimetric analysis experiment and is given 1.94 g 1.94 g of a contaminated mixture containing anhydrous magnesium chloride and potassium nitrate. to determine the percentage by mass of magnesium chloride in the mixture, excess silver nitrate is added to the mixture to precipitate the chloride ion as silver chloride. the mass of the silver chloride precipitate is found to be 1.43 g 1.43 g. which of the following is the mass percent of magnesium chloride in this sample?
a.25%
b.24%
c. 47%
d. 74%
Answers
The only material that will precipitate is silver nitrate. Silver chloride will precipitate as a result of the reaction between magnesium chloride and silver nitrate. 1.94 g 1.94 g of a contaminated combination including anhydrous potassium magnesium chloride and magnesium chloride is supplied to the subject of a gravimetric analysis experiment.
What is anhydrous magnesium?
The student uses extra AgNO3(aq) to precipitate the chloride ion as AgCl to determine the mixture's proportion by mass of MgCl2 (s). To ascertain the sodium chloride content, a gravimetric study is conducted. The ratio of magnesium chloride to silver nitrate is 1:2, meaning that a mineral water should use a minimum of two moles of silver nitrate while precipitating the chloride ions as silver chloride. Using gravimetric analysis, a student wants to ascertain the percentage of mass.
To learn more about potassium nitrate from given link
brainly.com/question/15080595
#SPJ4
HELLLP MEEEE
Write the chemical formula for each of the given compounds.

Answers
Answer:
sodium perchorate is NaCLO4
calcium sulfite CaSO3
potassium hydroxide KOH
lithium nitrate LiNO3
Explanation:
sodium perchorate has one Sodium
one Chlorine and 4 Oxygen
calcium sulfite has one Calcium on sulfate and 3Oxygen
lithium nitrate has one Lithium oneNitrogen
3 Oxygen
A solution of 49.0% H2SO4 by mass has a density of 1.39 g cm−3 at 293 K. A 22.6 cm3 sample of this solution is mixed with enough water to increase the volume of the solution to 88.5 cm3 .
Find the molarity of sulfuric acid in this solution.
Answers
Answer:
The molarity of the sulfuric acid in the solution is 1.77 M.
Explanation:
The molarity of the sulfuric acid in the solution can be found using the following equation:
\( C_{i}V_{i} = C_{f}V_{f} \rightarrow C_{f} = \frac{C_{i}V_{i}}{V_{f}} \)
Where:
\(C_{i}\): is the initial concentration of the acid
\(V_{i}\): is the initial volume of the solution = 22.6 cm³
\(V_{f}\): is the final volume of the solution = 88.5 cm³
The initial concentration of the H₂SO₄ is:
\( C_{i} = \frac{n}{V} = \frac{m}{M*V} = \frac{d*\% ^{m}_{m}}{M} \)
Where:
n: is the number of moles
m: is the mass
M: is the molar mass = 98.079 g/mol
d: is the density of the acid = 1.39 g/cm³
%: is the percent by mass = 49.0 %
\( C_{i} = \frac{1.39 \frac{g}{cm^{3}}*\frac{1000 cm^{3}}{1 L}*\frac{49 g}{100 g}}{98.079 \frac{g}{mol}} = 6.94 M \)
Finally, the final concentration of H₂SO₄ after the dilution is:
\( C_{f} = \frac{6.94 M*22.6 cm^{3}}{88.5 cm^{3}} = 1.77 M \)
Therefore, the molarity of the sulfuric acid in the solution is 1.77 M.
I hope it helps you!
(5). (10 points) Calculate the de Broglie wavelength (in m ) of (a). a mass of 50.0 g travelling at 0.2 m/s, (b). the same mass in (a) at 45 km/s (c). an He atom traveling at 1000 m/s (a typical speed at room temperature).
Answers
a. The de Broglie wavelength of the 50.0 g mass travelling at 0.2 m/s is \(6.626 * 10^{-32} m\).
b. The de Broglie wavelength of the 50.0 g mass travelling at 45 km/s is \(2.949 * 10^{-37} m.\)
c. The de Broglie wavelength of an He atom travelling at 1000 m/s is \(9.961 * 10^{-11} m.\)
The de Broglie wavelength (λ) of a particle can be calculated using the following formula: λ = h / p, where h is Planck's constant (\(6.626 * 10^{-34} J s\)) and p is the momentum of the particle, given by: p = m * v, where m is the mass of the particle and v is its velocity.
(a) For a mass of 50.0 g travelling at 0.2 m/s:
\(p = m * v = 50.0 g * 0.2 m/s = 10 g m/s\)
Converting the mass to kilograms:
m = 50.0 g = 0.0500 kg
Therefore,
\(p = 0.0500 kg * 0.2 m/s = 0.010 kg m/s\)
Using the de Broglie wavelength formula:
λ = \(h / p = 6.626 * 10^{-34} J s / 0.010 kg m/s = 6.626 * 10^{-32} m\)
Therefore, the de Broglie wavelength of the 50.0 g mass travelling at 0.2 m/s is \(6.626 * 10^{-32} m\).
(b) For the same mass travelling at 45 km/s:
Converting the velocity to m/s:
v = 45 km/s = 45,000 m/s
p = \(m * v = 0.0500 kg * 45,000 m/s = 2,250 kg m/s\)
Using the de Broglie wavelength formula:
λ = \(h / p = 6.626 * 10^{-34} J s / 2,250 kg m/s = 2.949 * 10^{-37} m\)
Therefore, the de Broglie wavelength of the 50.0 g mass travelling at 45 km/s is \(2.949 * 10^{-37} m.\)
(c) For an He atom travelling at 1000 m/s:
The mass of an He atom is approximately \(6.646 * 10^{-27} kg\).
\(p = m * v = 6.646 * 10^{-27} kg * 1000 m/s = 6.646 * 10^{-24} kg m/s\)
Using the de Broglie wavelength formula:
λ = \(h / p = 6.626 * 10^{-34} J s / 6.646 * 10^{-24} kg m/s = 9.961 * 10^{-11} m\)
Therefore, the de Broglie wavelength of an He atom travelling at 1000 m/s is \(9.961 * 10^{-11} m.\)
For more question on de Broglie wavelength click on
https://brainly.com/question/16595523
#SPJ11
The satellite image above shows the San Francisco area along the West Coast. What feature is marked by "X"?
A. A bay
B. A fresh water lake
C. A mountain
D. A volcano

Answers
A bag because it broad inlet of the sea where the land curves inwards
Look at the diagram of a fuel cell below.
Which part of the fuel cell does A represent?
air
anode
cathode
electrolyte
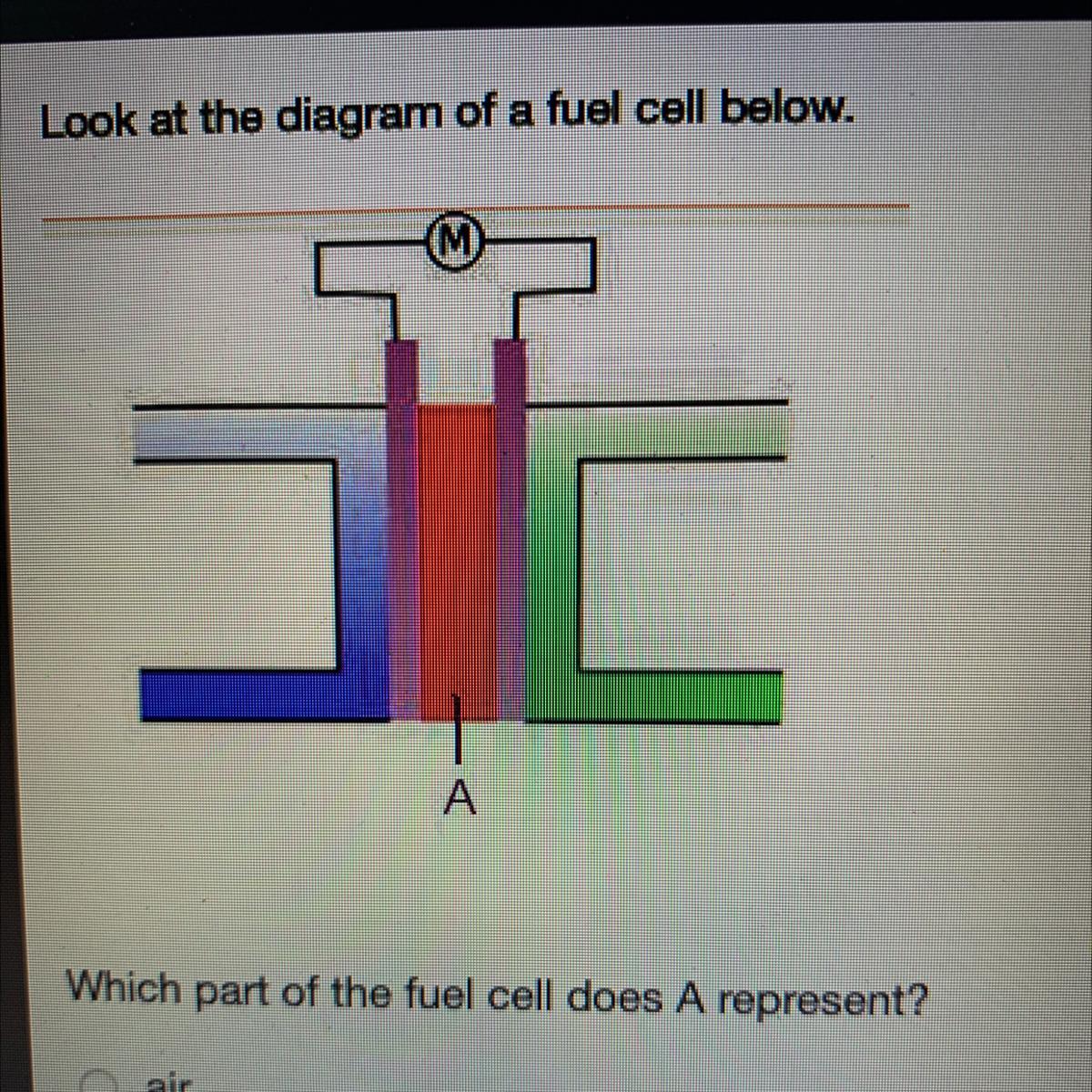
Answers
Answer:
I think its d) electrolyte
Explanation:
In the given diagram, The part of the fuel cell A represent is electrolyte.
What is Fuel Cell ?A fuel cell is the combination of two electrodes—anode (a negative electrode) and a cathode (positive electrode)— which is available around an electrolyte.
A hydrogen gas, is inserted to the anode, and air is inserted to the cathode.
Therefore, In the given diagram, The part of the fuel cell A represent is electrolyte.
Learn more about Fuel cell here ;
https://brainly.com/question/4607420
#SPJ5
Process by which plants, algae, and many types of bacteria use sunlight, water, and carbon dioxide to make food and oxygen
Answers
Answer: Photosynthesis
Explanation:
The process of photosynthesis is used to help make food for plants. The inputs are sunlight, water (H2O), and carbon dioxide (CO2). Then the outputs are glucose (C6H12O6) which is food, and oxygen (O2) is released.
I hope this helps :)
Part F Using your outline and the materials you've gathered, write a 250- to 500-word paper using word processing software. Be sure to proofread and revise your writing to catch any errors in grammar, spelling, logic, or organization. Add a works cited page at the end to give credit to your sources. Submit your completed paper and this activity to your teacher for evaluation. i need the answer please just make up a random story I REALLY NEED HELP
Answers
A wave is a recurring, periodic disturbance that moves from one place to another via a medium (like water).
What is wave?A wave is a disturbance that moves or propagates away from its source. Although waves can move energy between locations, they do not always move mass. Common examples of waves are light, sound, and ocean waves.
Mobile phones and radar systems are two noteworthy examples of wave applications. Even though radio waves are usually thought to be safe and contribute to background radiation, it is still advised to keep your distance from radio wave sources.
Read more about Wave
https://brainly.com/question/15663649
#SPJ1
complete question;
Using your outline and the materials you’ve gathered, write a 250- to 500-word paper using word processing software. Be sure to proofread and revise your writing to catch any errors in grammar, spelling, logic, or organization. Add a works cited page at the end to give credit to your sources. Submit your completed paper and this activity to your teacher for evaluation. This is for Unit Activity: Waves in edmentum
A light wave has a frequency of 5 x 1014 Hz and a speed of 3 × 10 m/s. Use
the wavelength equation to calculate the wavelength.
Answers
A light wave which has a frequency of 5 x 1014 Hz and a speed of 3 × 10 m/s has wavelength is 0.6 × 10^-6.
Wavelength is defined as the separation between two identical points (adjacent crests) in adjacent cycles as the signal travels through space or along a wire.The number of cycles or vibrations that a body in periodic motion experiences during one unit of time, as well as the number of waves that pass a fixed point in a unit of time is called Frequency. Frequency is expressed in Hertz (Hz).Speed of light, the rate at which light waves move through various substances. Specifically, the speed of light in a vacuum is presently calculated to be 3 × 10⁸ m/s.Frequency of light wave = ν = 5 × 10¹⁴ Hz
Speed of light c = 3 × 10⁸ m/s
we have to find out wavelength λ of light wave
We know that,
c = λ × ν
λ = c / ν
= 3 × 10⁸ / 5 × 10¹⁴
0.6 × 10^-6
Therefore, wavelength of light wave is
0.6 × 10^-6
Learn more about Wavelength here:
https://brainly.com/question/10750459
#SPJ9
Identify the unit that is used for atomic masses
Answers
Answer:
Explanation: Atomic weight is measured in atomic mass units (amu), also called daltons.
Which law states that the volume and absolute temperature of a fixed quantity of gas are directly proportional under
constant pressure conditions?
O Boyle's law
O Charles's law
O Dalton's law
O Gay-Lussac's law

Answers
Answer:
O Charles's law .
Explanation:
Hello!
In this case, since the use of gas laws leads to a good comprehension of how gases behave towards volume, pressure and temperature, we can review that the Boyle's law explains the pressure-volume variation, the Dalton's law the partial pressure effect, the Gay-Lussac's law that of pressure and temperature and the Charles' that of temperature and volume at constant pressure; thus, the answer for the asked question is:
O Charles's law
Best regards!
Why must the pH values of the mouth, stomach, and small intestine be different?
Answers
Answer:
so you don't digest your tounge
Two samples of carbon come into contact. A heat transfer will occur between sample A and sample B. What must be
true for heat to transfer from sample A to sample B?
O The average kinetic energy of A is greater than that of B.
O The average kinetic energy of B is greater than that of A.
O The average kinetic energy of both samples is equal.
O The average kinetic energy does not determine the direction of heat transfer.
Answers
The direction of heat transfer between two samples of carbon depends on their temperature difference, and not solely on their average kinetic energy. While the average kinetic energy of a substance is related to its temperature, it is not the determining factor for the direction of heat transfer.
When two samples of carbon come into contact, a heat transfer will occur between sample A and sample B. The direction of heat transfer is dependent on the temperature difference between the samples. Heat transfer always flows from a hotter object to a cooler object, so if sample A is hotter than sample B, heat will flow from A to B. If sample B is hotter than sample A, heat will flow from B to A.
The average kinetic energy of the molecules in a substance is related to its temperature. The higher the average kinetic energy, the higher the temperature of the substance. However, the average kinetic energy does not determine the direction of heat transfer.
It is possible for a substance with a lower average kinetic energy (and therefore a lower temperature) to transfer heat to a substance with a higher average kinetic energy (and therefore a higher temperature). This can occur if the substance with the lower temperature has a greater heat capacity, which means it can absorb more heat without a significant increase in temperature.
for more questions on kinetic energy
https://brainly.com/question/25959744
#SPJ8
The volume of a sample of water is 2.5 mL the volume of the sample in liters is
Answers
Answer:
0.0025Litters
Explanation:
2.5ml= 2.5x10^-3l
2.5ml= 0.0025l
Answer:
AAAAAAAA
Explanation:
Please help I’m really stuck:/
1. Use the equation weight = mg to find the weight of a 45 kg child.
2. Find the speed of a caterpillar that crawls a distance of 6.0 cm every
2.0 seconds. The equation for speed is v=d/t.
3. The circumference of a circle equals 2 mr, where r is the radius. Find
the circumference of a compact disc that has a radius of 6.0 cm.
2
HOLT SCIENCE SPECTRUM
Answers
Answer:
i would say two
Explanation:
I did the math
When two volumes of hydrogen gas react with one volume of oxygen gas, two
volumes of gaseous water are formed. Modify the diagram you made for #2 to
represent molecules of hydrogen, oxygen and water in this reaction
Answers
The closer to 7 a substance measures on the pH scale, the ______ the compound fill in the blank
Answers
Explanation:
If the PH is greater than 7 then the solution is basicIf the PH is less than 7 then the solution is acidicIf the PH is equal to 7 then the solution is neutralWhat happens to the electric current in a series circuit when voltage decreases
Answers
Which of the following describes an exothermic reaction?
O A. AHt, reactants > AHf, products
B. AHF, reactants = AHf, products
O C. AHf, reactants = 0
O D. AHF, reactants < AHt, products
Answers
The option A describes an exothermic reaction.
i.e, ΔHt,reactant > ΔHt, product
What is exothermic reaction?The reaction in which energy is release during the formation of reaction is called exothermic reaction.
Mathematically,
ΔHf = ΔH(product) - ΔH(reactant)
For exothermic reaction, ΔHt,reactant > ΔHt, product
So,for exothermic reaction, ΔHf is negative.
Where,
ΔHf = Enthalpy of formation of reaction
ΔH(product) = enthalpy of formation of product
ΔH(reactant) = enthalpy of formation of reactant
To learn more about exothermic reaction here.
https://brainly.com/question/10373907
#SPJ3
Sometimes a simple incident in our lives can actually have a deeper meaning and impact on us than might be apparent at the time. Write a reflective narrative about a memorable experience in your life.
Answers
I remember the day when I was walking to the grocery store and saw an elderly woman sitting on the sidewalk with her cart. She looked tired and was trying to catch her breath. At first, I walked past her, not wanting to get involved. But then something made me stop and turn around. I approached her and asked if she needed any help. She told me that she had been walking for a while and needed to rest for a bit. I helped her sit down on a nearby bench, and we started talking.
As we talked, I learned that her name was Mary, and she had lived in the neighborhood for over 50 years. She shared stories of her life and her struggles, and I listened with empathy. I could see the gratitude in her eyes, and it made me realize the power of human connection.
That experience taught me that we should never underestimate the impact of a simple act of kindness. It made me more aware of the people around me and more willing to help others in need. It also showed me the importance of empathy and how it can make a difference in someone's life.
Looking back, that moment was a turning point for me. It made me more compassionate and more aware of the needs of others. It showedme that a small gesture of kindness can have a ripple effect and make a big difference in someone's life. It also reminded me that we all have our own stories and struggles, and sometimes all we need is someone to listen and show us a little empathy.
Since that day, I have made it a point to be more aware of the people around me and to offer help whenever I can. Whether it's holding the door open for someone or offering a listening ear, I try to be more present and empathetic in my interactions with others.
That experience also taught me the importance of slowing down and taking the time to connect with people. In our fast-paced world, it's easy to get caught up in our own lives and forget about those around us. But by taking the time to connect with others, we can create meaningful relationships and make a positive impact in the world.
Overall, that simple encounter with Mary taught me a valuable lesson about the power of empathy and human connection. It's a lesson that I will always carry with me and strive to live by in my daily life.
In English, you're asked to write a reflective narrative about a life event with a deeper impact than initially visible. Describe the event, your reaction, its deeper meaning, and how it shaped you.
Explanation:This question pertains to reflective writing, a style typically used in English subject coursework. The task is about developing a narrative based on a memorable experience in your life that had a deeper impact than initially visible. Begin by selecting an event, which while appearing simple, had profound implications in retrospect. Describe the event in detail (who, what, where, when), discussing your personal reaction at the time. Then, explain its deeper meaning or the impact it had on you. Drawing conclusions about how this event shaped your perspectives or behaviours would constitute the reflective element of your narrative.
Learn more about Reflective Narrative here:https://brainly.com/question/33018651
#SPJ2
PLEASE TELL ME THE AWNSERS ITS A DOC FILE SO OPEN IT I WILL GIVE BRAINLIEST PLS HURRY
Answers
Determine whether each described process is endothermic or exothermic.
Wood burns in a fireplace Choose...
Ice melts into liquid water Choose...
Solid dissolves into solution, making ice pack feel cold Choose...
A process with a calculated positive q Choose...
A process with a calculated negative q Choose...
Acid and base are mixed, making test tube feel hot Choose...
Answers
Answer:
Following are the responses to the given choices:
Explanation:
Air woods is a smoking process as it releases heat.Incasereaction produces reaction energy, the response is then exothermic, while absorbs react energy, therefore the response is exothermic.Heat is essential for melting ice. Correspondingly, ice melts into liquid water as well as other reactions stop.Feel cold due to fuel absorption. That ice pack thus feels cold and brings another micro reaction to the stop.Unless the reaction's heat is positive, that process is endothermal.The reaction is exothermic unless the heat from the reaction is bad.Feel hot due to its loosening energy. A test tubefeelhot is, thus, an exothermic reaction.