oven mitts reduce which type of thermal energy transfer when you take a hot pan out of an oven
Answers
Answer:
conduction
Explanation:
Conduction is a form of thermal energy transferred through direct contact. Using an oven mitt reduces the amount of heat transferred:)
Answer:
The answer is Conduction.
Explanation:
I took AP3X quiz. Hope this helps.
Related Questions
Explain why the process of mining uranium involved in the use of nuclear energy has the greatest environmental impact of the entire process.
Answers
Uranium is a radioactive substance, which is extremely reactive. As a consequence, it cannot be found in the environment in its elemental state. During uranium, mining disturbances can influence both the quality and quantity of the surface water.
Several of the effects of uranium mining are identical to those experienced in other kinds of mining.
Due to the harmful effects of radioactive substances, the uranium mining for the use of nuclear energy has the greatest environmental influence.
a sample of gas occupies 4 liters at stp. the volume is changed to 2 liters and the temperasture is changed to 25 c. what us the new pressure of the gas?
Answers
The new pressure of the gas is 2.176atm. Boyle's Law will be applied to this issue. According to this rule, the pressure and volume fluctuate inversely when a gas is kept in a closed container and maintained at a constant temperature.
Given,
a sample of gas occupies 4 liters (V1)
the volume is changed to 2 liters (V2)
Temperature(T1) =25C
STP means p = 1 atm and T = 273.15 K
T2 = 25 + 273.15 = 298.15 K
The following is its mathematical expression:
p1V1 / T1 = p2V2 / T2
1 x 4 / 273.15 = p x 2 / 298.15
= 0.0146 = p*2/298.15
= 0.0146 *298.15 = 2p
2p = 4.352
therefore,
p = 4.352/2
p = 2.176
p = 2.176 atm
the new pressure of the gas is 2.176atm.
Learn more about pressure here:
https://brainly.com/question/19482357
#SPJ4
What type of radiation is emitted when polonium-212 forms astatine-212?
A: Alpha
B: Beta
C: Positron
D: Nuclear Fusion
Thanks:)
Answers
The type of radiation is emitted when polonium-212 forms astatine-212 is alpha radiations.
What is radioactive decay?When any unstable radioactive nuclei will emits any particle in the form of radiation to gain stability then the process is known as radioactive decay.
Polonium-212 is a unstable radioactive substance which will produces a daughter nuclei called astatine-212 through the radioactive decay, in which they emit the alpha particle in the form of radiations.
Hence the type of emitted radiation is alpha.
To know more about alpha decay, visit the below link:
https://brainly.com/question/17145324
#SPJ1
Answer:
beta
Explanation:
i took the test this is the answer 100%
Hemoglobin ___________. (Select all that apply).
returns carbon dioxide to your lungs to be exhaled
carries oxygen from your lungs to every cell in the body
is the most complex protein in your body
is not made up of amino acids
Answers
Answer:
Option B and A
Explanation:
First our lungs take purified oxygen from air then provide it to heartOur heart gives oxygen to hemoglobin which is present in our bloodIt carries oxygen to all cellsand comes back with cO_2most of the key energy-generating chemical reactions used by life on earth are
Answers
Most key energy-generating chemical reactions used by life on Earth Are processes like photosynthesis and cellular respiration.
Photosynthesis, primarily occurring in plants and some bacteria, converts sunlight, water, and carbon dioxide into glucose and oxygen.
Cellular respiration, utilized by both plants and animals, breaks down glucose and oxygen to produce ATP (adenosine triphosphate), which is a vital energy source for cells. Glycolysis, the Krebs cycle, and the electron transport chain are critical steps in cellular respiration.
These interconnected processes enable life forms to efficiently convert and store energy, supporting growth, reproduction, and various biological functions essential for survival.
Learn more about cellular respiration at
https://brainly.com/question/20069121
#SPJ11
3. In a neutral atom, this element has an atomic mass of 23 AMU and has 11 protons.
What element is it
Answers
The element in question is Sodium
An atomic mass unit is defined as 1/12 th of the mass of carbon-12 atom.
The mass of an atom is mainly due to its protons and neutrons in the nucleus.
Weight of a proton is 1.6 x 10^-27 kg
Weight of a neutron is 1.6 x 10^-27kg
However weight of an electron is 9 x 10^-31kg and hence is negligible in calculation of amu
It is given that weight is 23 AMU and number of protons is 11
The protons in a nucleus also act as atomic number of the periodic table
Proton number 11 is the atomic number of Sodium
Hence the element is Sodium
For further reference:
https://brainly.com/question/13668134?referrer=searchResults
#SPJ9
how does energy transfer from computer to speaker plz help fasttt
Answers
Answer:
As the coil moves through the magnetic field, created by the magnet, an electric current flows through it. The electric current flows out from the microphone to a computer or other recording device, and you can hear it. Aspeaker works in almost the same way, except that the process isreversed.
Explain how you could distinguish, without a flame test, between a solution that contains both Mg2+ and Ca2+ from one that contains only Mg2+.
Answers
One way to distinguish between a solution that contains both Mg²⁺ and Ca²⁺ from one that contains only Mg²⁺ without a flame test is by using a precipitation reaction.
A common reagent used for this purpose is sodium phosphate (Na₃PO₄), which reacts with Ca²⁺ions to form a white precipitate of calcium phosphate (Ca₃(PO₄)₂). Therefore, if a solution contains both Mg²⁺ and Ca²⁺, adding sodium phosphate to the solution will result in the formation of a white precipitate. On the other hand, if a solution contains only Mg²⁺, adding sodium phosphate will not result in any visible precipitate. By observing the presence or absence of a precipitate, we can distinguish between the two solutions.
To learn more about flame test https://brainly.com/question/22909071
#SPJ11
Match the following toxicity symptoms with the mineral with which they are associated.
Iron
Answers
Iron toxicity symptoms are generally associated with excessive intake or accumulation of iron in the body, which can lead to various health issues. These symptoms include.,
1. Fatigue and weakness: Excessive iron can cause damage to the mitochondria, which are responsible for producing energy in cells. This damage can result in reduced energy production, leading to fatigue and weakness.
2. Joint pain: Iron overload can cause inflammation in the joints, leading to pain and discomfort.
3. Abdominal pain: Excessive iron intake can lead to irritation of the gastrointestinal lining, causing abdominal pain.
4. Liver damage: High iron levels can lead to liver damage, including cirrhosis and liver failure.
5. Heart problems: Excess iron in the body can contribute to heart issues, such as arrhythmias, congestive heart failure, and even heart attacks.
6. Diabetes: High iron levels can affect insulin production and sensitivity, leading to an increased risk of developing type 2 diabetes.
7. Skin discoloration: Iron overload can cause an accumulation of iron in the skin, resulting in a bronze or gray discoloration.
Remember to monitor your iron intake and consult with a healthcare professional if you experience any of these symptoms.
Learn more about accumulation here
https://brainly.com/question/31814214
#SPJ11
name the structure in the figure in which an electron transport chain is located. describe the main function of the processes that occur in this structure.
Answers
The structure in the figure where an electron transport chain is located is the inner mitochondrial membrane.
The main function of the processes that occur in the inner mitochondrial membrane, specifically in the electron transport chain, is to generate ATP through oxidative phosphorylation.
The electron transport chain is a series of protein complexes embedded in the inner mitochondrial membrane. It plays a crucial role in the final stage of cellular respiration, which is the process by which cells extract energy from nutrients.
During oxidative phosphorylation, electrons are transferred through the electron transport chain from energy-rich molecules such as NADH and FADH2.
As electrons pass through the protein complexes, their energy is gradually released, and protons (H+) are pumped across the inner mitochondrial membrane from the mitochondrial matrix to the intermembrane space. This creates an electrochemical gradient.
The main function of this electron transport and proton pumping is to establish a proton motive force.
The gradient created by the electron transport chain drives the ATP synthase enzyme, located in the inner mitochondrial membrane, to produce ATP from ADP and inorganic phosphate. This process is known as chemiosmosis.
Overall, the electron transport chain in the inner mitochondrial membrane plays a crucial role in generating ATP, the energy currency of the cell, by utilizing the energy stored in the electrons derived from the breakdown of nutrients.
To know more about "Mitochondrial membrane" refer here:
https://brainly.com/question/31797295#
#SPJ11
Hydrated cobalt 2 chloride is heated to drive off the water. There are 13.31 grams of dry solid left from the original 17.00 gram of C o C l 2 dot H 2 O after heating. What is the formula of the hydrate?

Answers
If Hydrated cobalt 2 chloride is heated to drive off the water. The formula of the hydrate is: A. CoCl2.H2O.
What is formula of the hydrate?The formula of the hydrate can be determined using the law of constant composition.
Let x be the number of water molecules in the formula of the hydrate.
The formula of the hydrate can be represented as CoCl2.xH2O.
Both the mass of the dry solid (CoCl2) and the mass of the water (xH2O) can be calculated using the law of constant composition.
The mass of CoCl2 in the original 17.00 grams of hydrate is 13.31 grams, which is the mass of the dry solid after heating.
The mass of xH2O in the original 17.00 grams of hydrate is 17.00 - 13.31 = 3.69 grams.
The ratio of the masses of CoCl2 and xH2O is always constant, so we can set up the following proportion:
13.31 g / (1 mole CoCl2) = 3.69 g / (x moles H2O)
Dividing both sides by the molar mass of CoCl2 (110.98 g/mol), we get:
0.1203 moles / (1 mole CoCl2) = 3.69 g / (x moles H2O)
Dividing both sides by the molar mass of water (18.015 g/mol), we get:
0.1203 moles / (1 mole CoCl2) = 3.69 g / (x moles H2O) = 0.204 moles H2O / (x moles H2O)
So, x = 0.204 / 0.1203 = 1.69
Therefore, the formula of the hydrate is CoCl2.H2O.
Learn more about formula of the hydrate here https://brainly.com/question/25820990
#SPJ1
Answer:
it’s d
Explanation:
I have acellus
I NEED HELP PLEASE, THANKS! :)
The density of water at 4.00°C is 0.967 g/mL. How many molecules of water are present in a 499.8 mL bottle of water? Express your answer to the correct number of significant figures.
Answers
Answer:
\(\large \boxed{1.615 \times 10^{25}\text{ molecules water}}\)
Explanation:
You must calculate the mass of the water, convert it to moles, and then calculate the number of molecules.
1. Mass of water
\(\text{Mass } = \text{499.8 mL} \times \dfrac{\text{0.967 g}}{\text{1 mL}} = \text{483.3 g}\)
2. Moles of water
\(\text{Moles of water} = \text{483.3 g water} \times \dfrac{\text{1 mol water}}{\text{18.02 g water}} = \text{26.82 mol water}\)
3. Molecules of water
\(\text{No. of molecules} = \text{26.82 mol water} \times \dfrac{6.022 \times 10^{23}\text{ molecules water}}{\text{1 mol water}}\\\\= \mathbf{1.615 \times 10^{25}}\textbf{ molecules water}\\\text{The sample contains $\large \boxed{\mathbf{1.615 \times 10^{25}}\textbf{ molecules water}}$}\)
The number of molecules of water present in the bottle is 1.62×10²⁵ molecules.
We'll begin by calculating the mass of the water in the bottle.
Density of water = 0.967 g/mLVolume of water = 499.8 mLMass of water =?Mass = Density × Volume
Mass of water = 0.967 × 499.8
Mass of water = 483.3066 g
Finally, we shall determine number of molecules of water in the bottle.
From Avogadro's hypothesis,
1 mole of water = 6.02×10²³ molecules
But,
1 mole of water = 18 g
Thus, we can say that:
18 g of water = 6.02×10²³ molecules
Therefore,
483.3066 g of water = (483.3066 × 6.02×10²³) / 18
483.3066 g of water = 1.62×10²⁵ molecules
Thus, the number of molecules of water in the bottle is 1.62×10²⁵ molecules.
Learn more about Avogadro's number:
https://brainly.com/question/8933381
What are the purposes of theories in science?
b. What makes a theory a “good theory”?
please help
Answers
Answer:
Scientific theories are testable and make falsifiable predictions. They describe the causes of a particular natural phenomenon and are used to explain and predict aspects of the physical universe or specific areas of inquiry (for example, electricity, chemistry, and astronomy).
A good theory in the theoretical sense is (1) consistent with empirical observations; is (2) precise, (3) parsimonious, (4) explanatorily broad, and (5) falsifiable; and (6) promotes scientific progress (among others; Table 1.1).
Suggest two reasons why solid calcium has a greater density than solid potassium.
Any two of:
stronger metallic bonding
smaller ionic/atomic radius
two electrons per atom are delocalized
OR
greater ionic charge
greater atomic mass
Do not accept just "heavier" or "more massive" without reference to atomic mass.
[2 marks]
Answers
Two reasons why solid calcium has a greater density than solid potassium are:
Stronger metallic bonding
Smaller ionic/atomic radius
Stronger metallic bonding: Calcium has a stronger metallic bond between its atoms than potassium. This results in a more closely packed arrangement of atoms in solid calcium, leading to a greater density.
Smaller ionic/atomic radius: Calcium has a smaller ionic radius and atomic size compared to potassium. The smaller size of calcium atoms allows for a more closely packed arrangement of atoms in solid calcium, resulting in a greater density.
To know more about Calcium here:
https://brainly.com/question/29597119#
#SPJ11
If an asteroid has a semi-major axis of 73.4AU, then its orbital period in years carried out to 4 ) significant digits would be: Recall Kepler's 3
rd
law: a
AU
3
3=P
yr
2
If an asteroid has a semi-major axis of 73.4AU, then its orbital period in years carried out to 4 ) significant digits would be: Recall Kepler's 3
rd
law: a
AU
3
3=P
yr
2
Answers
The orbital period of the asteroid, carried out to 4 significant digits, is approximately 1652.0 years.
To find the orbital period (P) of an asteroid with a given semi-major axis (a), we can use Kepler's third law:
a³ = P²
Given that the semi-major axis (a) is 73.4 AU, we can substitute this value into the equation and solve for the orbital period (P):
(73.4 AU)³ = P²
(73.4)³ AU³ = P²
P² = (73.4)³ AU³
Taking the square root of both sides:
P = sqrt((73.4)³) AU
Using a calculator to evaluate the expression, we find:
P ≈ 1652.0 AU
Therefore, the orbital period of the asteroid, carried out to 4 significant digits, is approximately 1652.0 years.
Learn more about orbital period from the link given below.
https://brainly.com/question/31543880
#SPJ4
what is the chemical that had polluted flints drinking water supply
Answers
Answer:
Lead
Explanation:
While taking sample from flint's distillery, theybfoind 13000 ppb or parts per billion lead in the water itself
The fire preheats the surrounding materials and the growth rate increases.
(a) True
(b) False
Answers
Answer:
(a) True
Explanation:
The statement is true. In a fire, the heat generated by the flames preheats the surrounding materials, which can include combustible materials such as wood, paper, or fabric. The preheating of these materials increases their temperature, making them more susceptible to ignition and promoting the growth of the fire. This is one of the mechanisms by which a fire can spread and intensify.
Learn more about heat: https://brainly.com/question/17681868
#SPJ11
Workers digging a tunnel through a city find some
ancient pots decorated with geometric designs.
Which of the following tasks might they ask a
chemist to do? Explain your answer.
a. Determine the materials used to make the pots.
b. Explain what the designs on the pots represent.
c. Recommend how to store the pots to prevent
further damage.

Answers
Answer:
a. Determine the materials used to make the pots.
Explanation:
Workers digging a tunnel through a city who find some ancient pots decorated with geometric designs will employ the services of a chemist.
The chemist would help to determine the materials which were used to make the pots . This is usually done by various type of experiments and observations. After the constituent of the pot is gotten then more information would be derived from the possible source and use of the pot
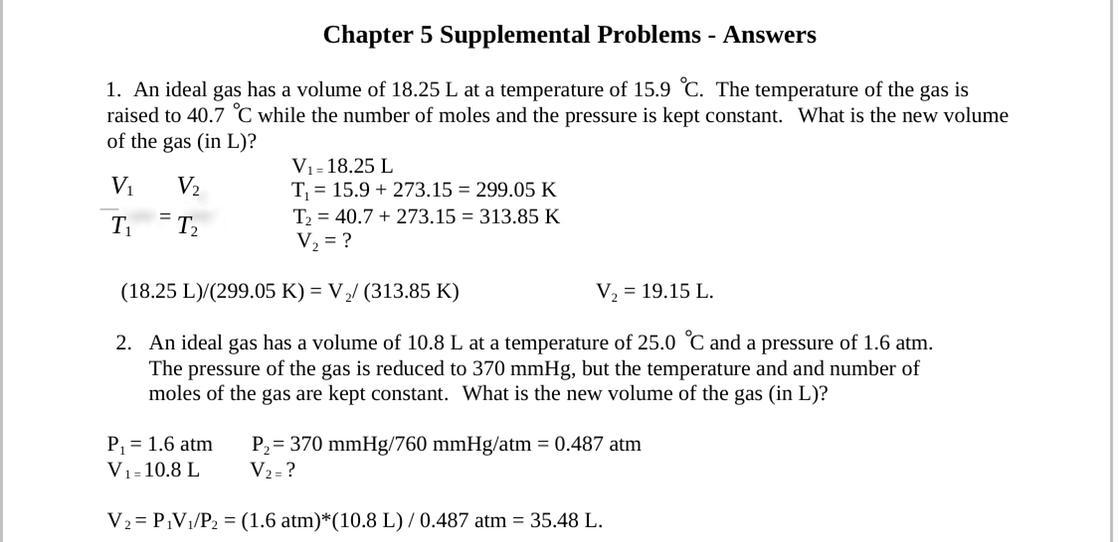
An ideal gas has a volume of 18.25 L at a temperature of 15.9 °C. The temperature of the gas is raised to 40.7 °C while the number of moles and the pressure of the gas are kept constant. What is the new volume of the gas (in L)?
B: An ideal gas has a volume of 10.8 L at a temperature of 25.0 °C and a pressure of 60 atm. The pressure of the gas is reduced to 370.0 mmHg, but the temperature and number of moles of the gas are kept constant. What is the new volume of the gas (in L)?
Answers
Answer:
V₂ = V₁ / T₁ * T₂ . If you prefer to set the final volume and want to estimate the resulting temperature, then the equation of Charles' law changes to: T2=T1/T1 multiplied by v^2.
2 moles of NO, was placed in an empty I dm' bottle and allowed to reach equilibrium according to the equation:
At equilibrium, 1.2 moles of N,O, dissociated. Calculate the value of the equilibrium constant for the reaction at that
temperature.
Answers
2NO(g) ⇌ N2(g) + O2(g)
According to the problem statement, 2 moles of NO were placed in a 1 dm^3 bottle and allowed to reach equilibrium, and at equilibrium, 1.2 moles of NO had dissociated. This means that the initial concentration of NO was:
[NO]initial = 2 mol / 1 dm^3 = 2 M
And the concentration of NO at equilibrium is:
[NO]equilibrium = (2 - 1.2) mol / 1 dm^3 = 0.8 M
Since the stoichiometry of the balanced equation is 2:1:1 for NO, N2, and O2, respectively, the equilibrium concentrations of N2 and O2 will also be 0.6 M.
The equilibrium constant (Kc) can be calculated using the equilibrium concentrations of the reactants and products, raised to the power of their stoichiometric coefficients. Therefore:
Kc = ([N2][O2]) / ([NO]^2)
Substituting the equilibrium concentrations into the equation, we get:
Kc = (0.6 M x 0.6 M) / (0.8 M x 0.8 M)
Kc = 0.5625
Therefore, the value of the equilibrium constant for the reaction at that temperature is 0.5625. Note that the units of Kc depend on the stoichiometry of the balanced equation. Since the stoichiometric coefficients are all 1, the units of Kc in this case are M^-1
What do LEGOs and DNA have in common?
Answers
Answer:
They are both building blocks
Explanation:
DNA builds up life while legos build things
A 0. 2500 g sample of a compound known to contain carbon, hydrogen and oxygen undergoes complete combustion to produce 0. 3664 g of co2 and 0. 1500 g of h2o. What is the empirical formula of this compound?.
Answers
The empirical formula of this compound CH₂O and the compound is Methanal
What is empirical formula?The molecular formula of a compound is the simplest integer ratio of the atoms present in the compound.
For the mass of C and H, using molar masses:
12.0 x (0.3664/44.0) = 0.096g C
2.02 x (0.15/18) = 0.016g H
As the sample is completely converted into CO₂ and H₂O.
So, 0.25g sample - 0.096g C - 0.016g H = mass of oxygen
Mass of oxygen = 0.138g
Using molar masses:
0.096g / 12.0 g/mol = 0.008 moles C
0.016g / 1.01 g/mol = 0.015 moles H
0.138g / 16.0 g/mol = 0.008 moles O
0.008 is lesser, so use it to normalize to find the ratio.
The ratios are:
0.008 moles C / 0.008 = 1 moles C
0.015 moles H / 0.008 = 1.8 moles H
0.008 moles O / 0.008 = 1 mole O
Moles of H = 1.8 ≈ 2
This makes the empirical formula as: CH₂O (Methanal)
To know more about empirical formula visit:
https://brainly.com/question/11588623
#SPJ4
When hydrochloric acid reacts with magnesium metal, hydro- gen gas and aqueous magnesium chloride are produced. what volume of 5.0 m hcl is required to react completely with 3.00 g of magnesium?
Answers
The volume of 5.0 m hcl is required to react completely with 3.00 g of magnesium is 20 L.
Chemical equation:Mg + 2HCl -----MgCl2 + H2
Given,
Molar mass of Mg = 24g/mol
Mole = Given mass/ Molar mass
Mole= 3/24
= 0.125 mol
From the given equation we get to know that
Mol ratio of Mg and HCl is 1:2.
Therefore,
mol of HCl = (2/1) × mol of Mg
=2× 0.125
= 0.25
Molarity = m× V
= M/m
= 5/0.25
= 20L
Thus, we find that the volume of 5.0 m hcl is required to react completely with 3.00 g of magnesium is 20 L.
learn more about Molarity:
https://brainly.com/question/26921570
#SPJ4
I NEED HELP WITH THIS ASAP!!!!!!!!!!!!!
Which chemical equation best represents the Law of Conservation of
Mass?
A. H+0 -> H20
B. H2 + 02 --> H20
C. 2H2 + 02 --> 2H,0
D. H2 + 02 --> 2H,02
Answers
Answer: The answer is B
Explanation:
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
the decomposition of hi(g)hi(g) at 298k298k is represented by the equilibrium equation above. when 100.torr100.torr of hi(g)hi(g) is added to a previously evacuated, rigid container and allowed to reach equilibrium, the partial pressure of i2(g)i2(g) is approximately 3.7torr3.7torr. if the initial pressure of hi(g)hi(g) is increased to 200.torr 200.torr and the process is repeated at the same temperature, which of the following correctly predicts the equilibrium partial pressure of i2(g)i2(g), and why?
Answers
PI2 ≈ 7.4 torrPI2 ≈ 7.4 torrs because it is directly proportional to the initial pressure of HIHI.
At equilibrium, the forward and reverse reactions occur at the same rate. Once equilibrium is reached, the amounts of each reactant and product remain constant. The equilibrium constant equation is a mathematical relationship that describes how the concentrations of products vary with the concentrations of reactants.
The state of a system whose properties are fixed under unvarying external conditions is called the equilibrium state. Chemical equilibria are dynamic in nature because reactants turn into products and products turn into reactants even after equilibrium is reached. However, the speed of forward and backward reactions is the same. Examples of balance include A book placed on the table. A car that moves at a constant speed. A chemical reaction in which the forward and reverse reaction rates are equal.
Learn more about The equilibrium here:-https://brainly.com/question/517289
#SPJ4
Distance and mass are factors that affect the force of
Answers
Answer:
Gravity is a fundamental underlying force in the universe. The amount of gravity that something possesses is proportional to its mass and the distance between it and another object.
Explanation:
Hope it helped, brainliest???
using standard heats of formation, calculate the standard enthalpy change for the following reaction. fe2o3(s) 2al(s) al2o3(s) 2fe(s)
Answers
As we know that ΔH Reaction o=ΔH fo (Products)−ΔH fo (Reactant)
⟹ΔH Reaction o =−1669−(−822)=−847 kJ/mol
As ΔH ReactioN o<0. So, the reaction is exothermic.
How do you calculate the standard enthalpy change for a reaction?
By deducting the total enthalpies of all the reactants from the total enthalpies of the products, the reaction enthalpy is determined. According to mathematics, tH is equal to the sum of the enthalpies of the reactants and the product.
The enthalpy of any reaction, as we already know, depends on the environmental physical parameters like temperature, pressure, etc. Any reaction's standard enthalpy may be determined when both the reactants and the products are present in their standard forms, which is the case for all reaction participants. The enthalpy change that takes place in a system when a substance undergoes a chemical reaction under normal circumstances is hence known as the standard enthalpy of reaction.
Conventionally, every substance's pure form at a pressure of 1 bar is considered to be its standard condition at a given temperature. For instance, liquid ethanol is considered to be at its standard state when it is 298 K and 1 bar of pressure
To be learn more about standard enthalpy refer to :
brainly.com/question/29556033
#SPJ4
Define matter and provide some examples of different states of matter
Answers
The three states of matter are solid - example is stone, liquid - example is water and gas - example is air.
What is matter?A matter is referred to as a substance which has a certain mass and takes up a certain volume in space.
For example pen, pencil, toothbrush, water, milk are matters as well as car, bus, bicycle is also a matter. So matter is considered as a living thing and a non-living thing.
There are three states of matter and they include;
solid - example is stoneliquid - example is watergas - example is airThey have different properties, which can be explained by looking at the arrangement of their particles. This is the theoretical temperature at which particles have the least amount of energy and the slowest movement.
Learn more about states of matter here: https://brainly.com/question/9402776
#SPJ1
(9) Which is true from the following ?
(A) Size of A1+ < Size of Al (B) Size of A1+ > Size of Al
(C) Size of F < Size of F (D) Size of Nat = Size of Na
(10) For which element the highest shielding effect for outermost electron is observed ?
(A) Element of group 13 and period 2 (B) Element of group 13 and period 3
(C) Element of group 13 and period 4 (D) Element of group 13 and period 5
(11) Which one is the descending order of atomic radius of elements of third period.
Na(Z = 11), Mg (Z = 12), Al (Z = 13) and Si(Z = 14) ?
(A) Si > Al > Mg > Na (B) Na > Mg > Al > Si
(C) Na < Mg < Al < Si (D) Na > Al > Mg >Si
(12) Which order is true with reference to size of species ?
(A) Pb < Pb2+ < Pb++
(B) Pb4! > Pb2+ > Pb
(C) Pb > Pb2+ > Pb4+
(D) Pb2+ < Pb < Pb4+
Answers
Answer:
The correct answer is A and D.
Explanation:
Please I want brainliest.
Reason:
The size of the ions follows the order:
Negatively charged ion > Neutral atom >
Positively charged ion
This is because of the size of ions increases when the electrons are added to the outermost shell of an atom.
This happens due to the increase in
electron-electron repulsion.
Moreover, when the electrons are removed from an atom, then the affective nuclear charge that is experienced by the outermost electron increases.
Thus the size of the positively charged ion is least.