of the following, which are characteristics of a reducing sugar?the sugar readily acts as an oxidizing agent
Answers
A reducing sugar is characterized by the presence of a free aldehyde or ketone group, its ability to undergo redox reactions, and its positive result in the Benedict's test. It is important to note that a reducing sugar acts as a reducing agent, not an oxidizing agent.
A reducing sugar is a type of sugar that readily acts as a reducing agent, not an oxidizing agent. It is capable of donating electrons to other substances, which allows it to undergo a redox reaction. This ability to donate electrons is due to the presence of a free aldehyde or ketone group in the sugar molecule.
Here are some characteristics of a reducing sugar:
1. Presence of a free aldehyde or ketone group: A reducing sugar has a free aldehyde (in the case of aldoses) or ketone (in the case of ketoses) group. This group is capable of reducing other substances by donating electrons.
2. Redox reactions: A reducing sugar can undergo redox reactions, where it donates electrons to another substance, thereby reducing it. This results in the oxidation of the sugar itself.
3. Benedict's test: A reducing sugar can be detected using a chemical test called the Benedict's test. This test involves heating the sugar with Benedict's reagent, which contains copper sulfate. If the sugar is a reducing sugar, it will reduce the copper sulfate to form a red precipitate of copper(I) oxide.
4. Examples: Some examples of reducing sugars include glucose, fructose, and maltose. These sugars have a free aldehyde or ketone group, allowing them to act as reducing agents.
Learn more about reducing sugar here:-
https://brainly.com/question/31596322
#SPJ11
Related Questions
Name the following compound
(ii) CH3COOCH2CH2CH3
Answers
Answer:
PROPYL ETHANOATE (OR PROPYL ACETATE)
Explanation:
1st step: Identify the functional group of the compound.
COO is present, hence it is an ester.
2nd step: Split up the compound into CH3COOH and CH3CH2CH2OH.
Comes from ethanoic acid and propanol
3rd step: Join the names of the alcohol and the carboxylic acid together.
Hence, propyl ethanoate.
A mole ratio is used to convert the given number of moles of one substance to the unknown number of moles of a second substance. True or False?
Answers
True. A mole ratio is a conversion factor used to relate the number of moles of one substance in a chemical reaction to the number of moles of another substance involved in the same reaction.
The mole ratio is determined by the stoichiometry of the balanced chemical equation and specifies the proportional relationship between the number of moles of reactants and products involved in the reaction. By using mole ratios, it is possible to convert the given number of moles of one substance into the corresponding number of moles of another substance, allowing for stoichiometric calculations to be performed.
Find more about mole ratio
brainly.com/question/28298591
#SPJ4
Products do what? what do products do in science
Answers
Answer:
In chemistry, a product is a substance that is formed as the result of a chemical reaction. In a reaction, starting materials called reactants interact with each other.
Explanation:
Hope this helps and good luckk :)
The following irreversible reaction A-3R was studied in the PFR reactor. Reactant pure A (CAO=0.121 mol/lit)is fed with an inert gas (40%), and flow rate of 1 L/min (space velocity of 0.2 min-1). Product R was measured in the exit gas as 0.05 mol/sec. The rate is a second-order reaction. Calculate the specific rate constants.
Answers
The specific rate constant of the second-order irreversible reaction is 122.34 L/mol.s.
A second-order irreversible reaction A-3R was studied in a PFR reactor, where reactant pure A (CAO=0.121 mol/lit) is fed with an inert gas (40%), and flow rate of 1 L/min (space velocity of 0.2 min-1). Product R was measured in the exit gas as 0.05 mol/sec.
To calculate the specific rate constant, we use the following equation:0.05 mol/sec = -rA * V * (1-X). The negative sign is used to represent that reactants decrease with time. This equation represents the principle of conservation of mass.Here, V= volume of the PFR. X= degree of conversion. And -rA= the rate of disappearance of A= k.CA^2.To calculate the specific rate constant, k, we need to use a few equations. We know that -rA = k.CA^2.We can also calculate CA from the volumetric flow rate and inlet concentration, which is CAO. CA = (CAO*Q)/(Q+V)The volumetric flow rate, Q = V * Space velocity (SV) = 1 * 0.2 = 0.2 L/min.
Using this, we get,CA = (0.121*0.2)/(1+0.2) = 0.0202 mol/LNow, we can substitute these values in the equation of rate.0.05 = k * (0.0202)^2 * V * (1 - X)The volume of PFR is not given, so we cannot find the exact value of k. However, we can calculate the specific rate constant, which is independent of volume, and gives the rate of reaction per unit concentration of reactants per unit time.k = (-rA)/(CA^2) = 0.05/(0.0202)^2 = 122.34 L/mol.
Learn more about specific rate constant:
https://brainly.com/question/33346381
#SPJ11
which representation is best at getting a quick overview of the number of each element in a compound
Answers
The chemical formula is the best representation for obtaining a quick overview of the number of each element in a compound. A chemical formula is a symbolic representation of a compound's chemical composition that indicates the number and type of atoms present in the compound. In general, chemical formulas use chemical symbols and numerical subscripts to express the composition of a compound.
The chemical symbols represent the atoms of each element in the compound, while the numerical subscripts indicate how many atoms of each element are present. A chemical formula can be represented in a variety of ways. The most common form of a chemical formula is empirical formula, which expresses the relative number of each element in the compound in the lowest integer ratios. Another way of representing a chemical formula is a molecular formula, which provides the exact number of atoms of each element in the compound.
To know more about integer ratios visit
https://brainly.com/question/24055119
#SPJ11
1. A sample of nitrogen occupies a volume of 250 mL
at 298 K. What volume will it occupy at 268 K?
Answers
Answer:
0.225 L = 225 mL
Explanation:
Charles' Law states that for a fixed pressure, the volume is directly proportional to the temperature, which means things expand at the same rate as the rate of it being heated up
Mathematically, we can write that as:
V1/T1 = V2/T2, where 1 and 2 are the two situations
So, when we substitute the values in, we get:
0.25 L/298 K = V2/268 K
Rearranging this, we get:
(0.25/298) x 268 = 0.225 L = 225 mL.
This also makes sense, as the volume reduced a little when the temperature reduced (cooled down)
Let me know if this makes sense, and hope I helped! :)
Which elements are malleable and good conductors of electricity.
Answers
Answer:
metals are malleable and good conductors of electricity.
Calcium carbide, CaC₂ reacts with water to form ethyne, C₂H₂, and calcium hydroxide.
the equation for the reaction is shown.
CaC₂(s) + 2H₂O(l)→ C₂H₂(g) + Ca(OH)2(s)
which Volume of ethyne is produced when 6g of water react completely with calcium carbide?
A. 4dm^3
B. 8dm^3
C. 36dm^3
D. 72dm^3
( please solve explanation!! )
Answers
Answer:
36dm³
Explanation:
For this question we are required to find the volume of the ethyne which is released while reacting 6 grams of water with calcium carbide.
Here to find the volume of ethyne produced, we can find the moles of the ethyne in the reaction by using the mole concept, and then converting the mol into the volume using the formula V=nRT/p or PV=nRT (n being the moles of the gas)
Select all intermolecular forces that contribute to creating a solution of HCl in CH3CN.
Group of answer choices
O London Dispersion
O Dipole-dipole
O H-bonding
Answers
Dipole-dipole and London dispersion forces are the two intermolecular interactions found in HCl in CH₃CN. The dipole-dipole forces are stronger of the two. The H-Cl bond dipole is what creates the dipole-dipole forces (as Cl is more electronegative than H) (option 1 & 2)
A polar molecule is CH₃CN. Dipole-dipole interaction is the intermolecular force at work in this molecule. This molecule does not have any hydrogen bonds, and dipole-dipole interactions are weaker than hydrogen bonds.
Because the chlorine atom has a tiny negative charge and the hydrogen atom has a slight positive charge, HCl molecules, for instance, have a dipole moment. There is a tiny dipole-dipole force of attraction between nearby HCl molecules as a result of the force of attraction between oppositely charged particles.
Learn more about dipole-dipole interaction here: https://brainly.com/question/11828970
#SPJ4
the elements of group 15, 16, 17 are less reactive as they go down in the group of modern perodic tale. why ?
Answers
Answer: This is because the atomic radius increases with increasing electronic energy. This reduces the attraction for the valence electrons of other atoms, reducing reactivity.
how many atoms are in 7.2mol of sodium? step by step answer
Answers
Answer:
4.34 times 10^24
Explanation:
number of atoms=7.2 times Avogadro's number
=7.2 times 6.02 times 10^23
=4.34 times 10^24
Answer:
Explanation: we will multiple the amount of moles to the Avogadro's number for example:
7.2*6*10 to the power 23
Chlorophyll molecules in chloroplasts normally only fluoresce a very small amount compared to chlorophyll that has been extracted into a solvent solution. True or false?.
Answers
Chlorophyll molecules in chloroplasts normally only fluoresce a very small amount compared to chlorophyll that has been extracted into a solvent solution, true.
What is a chlorophyll?A green pigment found in the mesosomes of cyanobacteria and in the chloroplasts of algae and plants is known as Chlorophyll Chlorophyll is derived from the two Greek words khloros which means "pale green" and phyllon means "leaf". Chlorophyll allow plants to absorb energy from light.Joseph Bienaimé Caventou and Pierre Joseph Pelletier in 1817 first isolated and named Chlorophyll. The presence of magnesium was discovered in 1906 in chlorophyll and that was element's first detection in living tissueChlorophyll a and b are the two types of chlorophyll that exist in the photosystems of green plants.To learn more about chlorophyll: https://brainly.com/question/13500580
#SPJ4
4. Assign an oxidation state to the element whose atom is underlined in the following compound: AIH3
O Al: +5
O Al: -5
Ο Ο Ο
Al: -3
O Al: +3
Answers
Answer: The oxidation state of Al in \(AlH_{3}\) is +3.
Explanation:
The number attained by an atom or substance due to loss or gain of electrons is called oxidation state.
For example, in \(AlH_{3}\) it is known that the oxidation state of hydrogen is -1 as aluminum is a cation so it cannot have an oxidation state as 'minus'.
Whereas hydrogen has the property to act both as a cation and an anion.
Let us assume the oxidation state of Al is x in the compound \(AlH_{3}\).
Hence, oxidation state of Al is calculated as follows.
\(x + 3(-1) = 0\\x - 3 = 0\\x = +3\)
This means that the oxidation state of Al in \(AlH_{3}\) is +3.
Thus, we can conclude that oxidation state of Al in \(AlH_{3}\) is +3.
What is a good example of ACCURACY and PRECISION? *
Answers
Answer:
Bow and arrow
Explanation:
A bow and arrow need accuracy to hit a target. It needs precision to hit the small target
Calculate the approximate volume of a 0.600 mol sample of gas at 15 °C and a pressure of
1.0 atm.
Answers
1) List known values.
sample: 0.600 mol
Pressure: 1.0 atm
Temperature: 15ºC
Constant: 0.082057 L⋅atm⋅K−1⋅mol−1
List unknown values
Volume:
2) Set the equation and solve for V.
\(PV=\text{nRT}\)\(\frac{PV}{P}=\frac{nRT}{P}\)\(V=\frac{nRT}{P}\)3) Converting to proper units.
15ºC + 273.15 = 288.15 K
4) Plug in known values.
n: 0.600 mol
R: 0.082057 L⋅atm⋅K−1⋅mol−1
T: 288.15 K
P: 1.0 atm
\(V=\frac{(0.600\text{mol)}(0.082057L\cdot\text{atm}\cdot K^{-1}\cdot mol^{-1})(288.15\text{ K)}}{1.0\text{ atm}}=14.18683\text{ L}\)The sample will occupy a volume of 14.19 L.
manganese (IV) nitride formula
Answers
Answer:
Mg(NO3)2 is the formula of manganese (IV) nitride.
The formula for Manganese (IV) nitride is Mn3N4.
What is the formula for Manganese (IV) nitride?Manganese (IV) nitride is represented by the chemical formula Mn3N4. It consists of three manganese atoms (Mn) and four nitrogen atoms (N). The Roman numeral IV indicates that manganese has a +4 oxidation state in this compound.
Its powder can be used in the preparation of other nitride compounds with high hardness, high thermal conductivity, corrosion resistance, wear resistance and high temperature resistance properties.
Read more about manganese
brainly.com/question/28533522
#SPJ6
Not sure how to do these, one example would prolly help me do the rest

Answers
Answer:
3. a. I⁻
Explanation:
The charge of the ion is determined by which group (vertical on the periodic table) the element is in. Iodine is in the halogens and therefore is most likely to have a 1- charge.
What would happen if N2 were added to N2(g) + O2(g) = 2NO(9) at
equilibrium?
O A. Keq would decrease
O B. More No would form.
O C. More O2 and No would form.
O D. More N2 and Oz would form.
Answers
Balanced chemical equation is :
\(N_2(g)+O_2(g)-->2NO(g)\)
It is given that the equation is in equilibrium.
We need to find what will happen if we add more \(N_2\) is added .
By Le Chatelier's principle :
Changing the concentration of a chemical will shift the equilibrium to the side that would counter that change in concentration.
It means production of the side where content is added will decrease and concentration on other side will increase .
So , more NO would form .
Therefore, option B. is correct.
Hence, this is the required solution.
The study of chemicals is called chemistry.
The correct answer is B.
According to the principle, the reaction goes from the higher side to the lower side.
According to the question, N2 is added to the reaction which will lead to an increase in the amount of N2 in the reactant.
More amount in reactant will lead to the formation of more product and the in the equation more NO will form.
Hence, the correct answer is B that is More No would form.
For more information, refer to the link:-
https://brainly.com/question/25305623
Weathering of Earth's surface can have which of the following effects? *
8 points
Building mountains
Creating hurricanes
Breaking down rocks
Causing lava flow
Answers
For each bond, predict region where you will expect an IR absorption.
Answers
To predict the regions where you can expect IR absorption for each bond, we need to consider the functional groups present and their respective vibrational frequencies.
Here are some common functional groups and their approximate IR absorption ranges:
O-H (alcohol or phenol): 3200-3600 cm⁻¹ (broad) N-H (amine or amide): 3100-3500 cm⁻¹C-H (alkane, alkene, or aromatic): 2800-3300 cm⁻¹C=O (carbonyl, such as ketone, aldehyde, or ester): 1650-1750 cm⁻¹C=C (alkene or aromatic): 1600-1680 cm⁻¹C≡N (nitrile): 2210-2260 cm⁻¹C≡C (alkyne): 2100-2250 cm⁻¹ (usually weak)These ranges will help you predict the regions where IR absorption is expected for various types of bonds. Make sure to analyze the molecule's structure and identify the functional groups present to determine the expected IR absorptions.
Learn more about IR absorption at https://brainly.com/question/29746356
#SPJ11

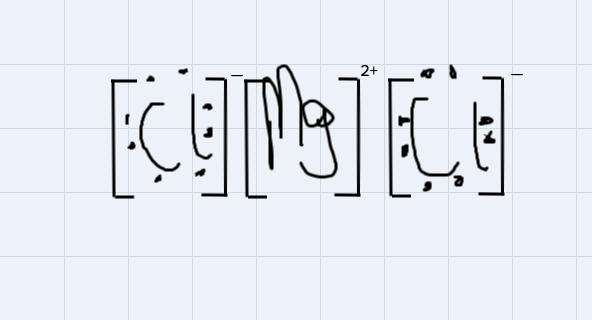
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.
Which situation shows a nonrenewable resource in use?
O A. Lobsters are caught off the coast of Maine.
B. Kaolin is used to make porcelain.
C. Solar energy reaches Earth's surface.
O D. Trees re-seed after a forest fire.
SL
Answers
Answer:
B
Explanation:
According to the bohr model of the atom, the single electron of a hydrogen atom circles the nucleus?
Answers
According to the bohr model of the atom, the single electron of a hydrogen atom circles the nucleus is specific allowed orbits.
What is orbits?Encyclopedic entry. An orbit is a regular, repeating path that one object takes around another object or center of gravity. Orbiting objects, which are called satellites, include planets, moons, asteroids, and manmade devices.An orbit is a regular, repeating path that one object takes around another object or center of gravity. Orbiting objects, which are called satellites, include planets, moons, asteroids, and manmade devices.Objects orbit each other because of gravity. Gravity is the force that exists between any two objects with mass. Every object, from the smallest subatomic particle to the largest star, has mass. The more massive the object, the larger its gravitational pull. Gravitational pull is the amount of force one object exerts on another object.
To learn more about Gravitational refer to:
https://brainly.com/question/27943482
#SPJ4
ferrous iron (fe2 ) oxidation generally occurs in environments with a. highoxygencontent b. alkalineconditions c. acidic conditions o d. littleornolightpresent
Answers
Ferrous iron\((Fe2+)\)oxidation generally occurs in environments with high oxygen content. The correct option is a.
This is because the oxidation process involves the conversion of ferrous ions \((Fe2+)\) to ferric ions \((Fe3+)\)through a reaction with oxygen. Oxidation is the process by which a substance loses electrons.
Ferrous iron \((Fe2+)\) oxidation involves the loss of two electrons. Ferrous iron can be oxidized by a variety of substances, including oxygen, nitrate, and manganese. However, it is most commonly oxidized by oxygen.
The rate of ferrous iron oxidation depends on a number of factors, including temperature, pH, and the presence of other chemicals.
In general, ferrous iron oxidation occurs more quickly in environments with acidic conditions.
This is because the hydrogen ions in acidic solutions can react with ferrous iron to form ferric iron \((Fe3+)\), which is more stable and less soluble than ferrous iron. As a result, it precipitates out of the solution, which makes it easier to remove.
to know more about oxidation refer here:
https://brainly.com/question/9347985#
#SPJ11
.
14. All living things react to sound, touch etc. Whatever causes a living thing to react is called a stimulus. The reaction is called a response. Which of these is an example of a STIMULUS- RESPONSE?
Answers
Balance the following equation using the change in oxidation numbered method Ag + H2S+ O2 —> Ag2 S + H2O
Answers
Answer
Explanation
In the oxidation number method of balancing chemical equations, you determine the oxidation numbers of all atoms. Then you multiply the atoms that have changed by small whole numbers. You are making the total loss of electrons equal to the total gain of electrons. Then you balance the rest of the atoms.
The given equation is:
Ag + H₂S + O₂ —> Ag₂S + H₂O
Step 1. Identify the atoms that change oxidation number.
Left hand side: Ag = 0, H = +1, S = -2, O = 0
Right hand side: Ag = +1, S = -2, H = +1, and O = -2
The changes in oxidation number are:
Ag: 0 → +1; Change = +1
O: 0 → -2; Change = -2
Step 2. Equalize the changes in oxidation number.
Each Ag atom has lost one electron, and each O atom has gained two electrons.
O: 0 → -2; Change = -2,
what factors determine the amount of radiant energy we will receive on earth?
Answers
a molecule has two bonded atoms around the central atom. the central atom does not have any lone pairs. what is the geometry of the molecule?
Answers
a molecule has two bonded atoms around the central atom. the central atom does not have any lone pairs. The molecule has a linear geometry.
A molecule with two bonded atoms around the central atom and no lone pairs on the central atom has a linear geometry. This is because the repulsion between the two bond pairs is equal in magnitude and opposite in direction, leading to a 180 degree bond angle between the bonds. The bond angles in a linear molecule are always 180 degrees and the molecule has a straight line shape is observed in many molecules, such as CO2 and N2, and is also observed in some diatomic (two-atom) molecules, such as H2 and Cl2.
Learn more about linear geometry here:
https://brainly.com/question/16178099
#SPJ4
Which substance cannot be separated or broken down into simpler substances by chemical means?
table salt
gold wire
candle wax
water vapor
Answers
Answer: Gold Wire
Explanation:
Out of all of these options only gold wire is an element, elements are pure and cannot be broken down by any chemical means
hope it helped, good luck :)
what is the molarity of a 350 mL solution of NH4NO3 containing 0.56 moles of solute
Answers
1.6M is the molarity of a 350 mL solution of NH\(_4\)NO\(_3\) containing 0.56 moles of solute. Molarity is also known as concentration.
Molarity is also known as concentration in terms of quantity, molarity, or substance. It is a way to gauge how much of a certain chemical species—in this case, a solute—is present in a solution. It is a substance every volume of solution in terms of quantity.
The measurement of moles / litre is the molarity unit that is most frequently used in chemistry. One mol/L of a solution's concentration is referred to as its molarity. It is frequently abbreviated as 1 M.
molarity = number of moles/ volume of solution in liter
= 0.56×1000/ 350
= 1.6M
To know more about Molarity, here:
https://brainly.com/question/8732513
#SPJ1