Answers
Answer:
I think it B
Explanation:
Related Questions
how many moles of each reactant are needed to produce 3.60*10 to the power of 2 g ch3oh
Answers
We need 5.62 moles of H2 and 5.62 moles of CO to produce 3.60 × 10^2 g of CH3OH.
How to calculate the mole ?
To calculate the number of moles of a substance, we use the formula:
moles = mass / molar mass
where "mass" is the mass of the substance in grams and "molar mass" is the molar mass of the substance in grams per mole.
To determine the number of moles of reactants needed to produce a given amount of product, we need to use the balanced chemical equation for the reaction and the molar mass of the product.
Assuming that the reaction is:
2H2 + CO → CH3OH
We can see that the stoichiometry of the reaction is 2:1, which means that for every 2 moles of H2, we need 1 mole of CO to produce 1 mole of CH3OH.
The molar mass of CH3OH is:
12.01 + 4(1.01) + 16.00 = 32.04 g/mol
Therefore, to produce 3.60 × 10^2 g of CH3OH, we need:
n(CH3OH) = (3.60 × 10^2 g) / (32.04 g/mol) = 11.23 mol
Since the stoichiometry of the reaction is 2:1, we need half as many moles of H2 as we do of CH3OH:
n(H2) = 1/2 × n(CH3OH) = 1/2 × 11.23 mol = 5.62 mol
And we need half as many moles of CO as we do of CH3OH:
n(CO) = 1/2 × n(CH3OH) = 1/2 × 11.23 mol = 5.62 mol
Therefore, we need 5.62 moles of H2 and 5.62 moles of CO to produce 3.60 × 10^2 g of CH3OH.
To know more about moles visit :-
https://brainly.com/question/1634438
#SPJ1
For ideal gases, the molecules themselves ___________, which is one assumption of the ideal gas law.
Answers
For ideal gases, the molecules themselves have negligible volume, which is one assumption of the ideal gas law.
Ideal gases, the molecules themselves are assumed to have negligible volume. This means that the gas particles are considered to be point masses with no physical size or volume.
This assumption is made because the volume occupied by the gas molecules is assumed to be insignificant compared to the total volume of the gas sample.
It simplifies calculations and allows for the application of the ideal gas law, which relates pressure, volume, temperature, and the number of moles of gas.
Although real gases do have finite molecular volumes, the assumption of negligible volume is valid under conditions of low pressure and high temperature, where intermolecular interactions are minimal.
To know more about ideal gases refer here
https://brainly.com/question/11951894#
#SPJ11
how can you test a sample of soil for acidity or alkalinity
Answers
Answer:
Scoop a soil sample into a fresh container, add 1/2 cup of water, and mix. Then, add 1/2 cup of baking soda. If the soil bubbles or fizzes, the soil is acidic. The reaction you're seeing is the result of acidic soil coming into contact with an alkaline substance (baking soda).
Explanation:
Hope this helped!
Xà phòng hoá hoàn toàn 22,2g hỗn hợp gồm 2 este HCOOC2h5 và ch3cooch3 bằng dung dịch naoh 1m ( đun nóng) thể tích dung dịch naoh tối thiểu cần dùng là
Answers
Answer:
I can't understand your language? can you write in English
I'm sorry
A study was conducted of 90 adult male patients following a new treatment for congestive heart failure. One of the variables measured on the patients was the increase in exercise capacity (in minutes) over a 4-week treatment period. The previous treatment regime had produced an average increase of μ=2 minutes. The researchers wanted to evaluate whether the new treatment had increased the value of μ in comparison to the previous treatment. The data yielded y(bar)=2.17 and s=1.05.
(a) if the actual value of mu is 2.1 and alpha is reduced from 0.05 to 0.01, what would be the effect on the power curve?
(b) If the sample size is reduced from 90 to 50, what would be the effect on the power curve?
Answers
a. Decreasing alpha from 0.05 to 0.01 makes the significance level more stringent. You will be less likely to reject the null hypothesis, even when it's false. This increases the probability of a Type II error, thus potentially reducing the power of the test. The power curve will shift to the left.
b. If the sample size is reduced from 90 to 50, the effect on the power curve is that it will also shift towards the left.
What more should you know about decreasing the alpha and the power curve?The power curve is a graph that shows the probability of rejecting the null hypothesis as a function of the true value of the mean.
In the given scenarios of this study, Reducing the significance level and reducing the sample size will shift the power curve to the left, indicating a decrease in the statistical power of the test.
The power of a statistical test is the probability that it correctly rejects the null hypothesis when the alternative hypothesis is true.
a) Reducing alpha from 0.05 to 0.01 means that we are more stringent in our assessment of whether the new treatment is effective.
This will result in a decrease in the power of the test, meaning that it is less likely that we will be able to detect a difference between the new treatment and the previous treatment.
b) If the sample size is reduced from 90 to 50, the effect on the power curve is that it will also shift towards the left.
This is because a smaller sample size decreases the power of the test. A larger sample size provides more information and thus makes it more likely to correctly reject the null hypothesis when the alternative hypothesis is true.
Therefore, by reducing the sample size, you are decreasing the likelihood of detecting a true effect if one exists, thus reducing the power of the test.
Find more exercises on alpha level in a study;
https://brainly.com/question/6372035
#SPJ4
convert eachh into scientific notation
4.060 x 10^5 →
7 x 10^3 →
5.0 x 10^-4 →
8 x 10^-2 →
Answers
Answer:
0.00580 →
3000 →
0.000908 →
200. →
Explanation:
yay
Answer:
\(\large \boxed{\mathrm{view \ explanation}}\)
Explanation:
4.060 × 10⁵ (scientific notation)
The decimal point moves 5 places to the right.
⇒ 406000 (standard form)
7 × 10³ (scientific notation)
The decimal point moves 3 units to the right.
⇒ 700 (standard form)
5.0 × 10⁻⁴ (scientific notation)
The decimal point moves 4 units to the left.
⇒ 0.0005 (standard form)
8 × 10⁻² (scientific notation)
The decimal point moves 2 units to the left.
0.08 (standard form)
Juan's mother drives 7.25 miles southwest to her favorite shopping mall. A. Average velocity is the total displacement divided by the total time taken. What is the average velocity of Juan's mother's automobile if it arrives at the mall in 20.0 minutes? B. Does the average velocity reflect the how fast Juan's mother was driving at every point in her journey? Explain your answer by comparing the terms average velocity and instantaneous velocity.
Answers
Answer:
See explanation
Explanation:
Average velocity = distance traveled/time
distance traveled = 7.25 miles
Time taken = 20 minutes or 0.33 hours
Average velocity = 7.25 miles/0.33 hours = 21.97 miles per hour
This average velocity does not reflect how fast Juan's mother drove at every point in the journey.
At every point in the journey, Juan's mother had an instantaneous velocity given by the velocity at that given instant divided by time taken up to that point.
The instantaneous velocity gives the velocity at particular instants throughout the journey while the average velocity reflects the average velocity of the entire journey.
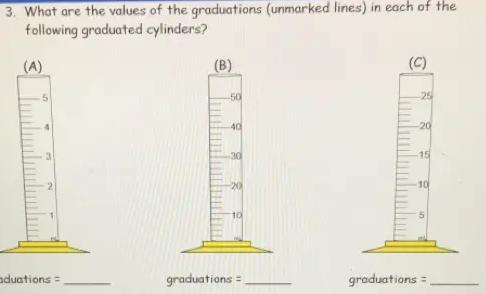
3. What are the values of the graduations (unmarked lines) in each of the
following graduated cylinders?
Q
(A)
50
3
graduations =
(B)
50
-40
-30
-20
10
graduations =
(C)
25
-20
-15
10
5
graduations =
Answers
The values of the Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
Graduated Cylinder - A popular piece of scientific equipment used to measure the volume of a liquid is a graduated cylinder, sometimes referred to as a measuring cylinder or mixing cylinder. Its form is slender and cylindrical. Each marked line on the graduated cylinder indicates the volume of liquid that has been measured.
The clear graduated cylinder is widely used for volume measurements and is regarded to be more accurate than a beaker since it contains permanently marked incremental graduations.
To calculate the value of graduations-
1. Subtract the two values to get the value between the labeled graduations.
2. Determine how many spaces there are between the two graduations. Keep in mind that volume equals space.
3. Divide the number of gaps by the value between the graduations.
For cylinder A.
2 ml- 1ml = 1ml
Number of spaces = 5
∴1/ 5 = 0.2
Graduation = 0.2ml
For cylinder B -
20-10 = 10
number of spaces = 5
∴10/ 5 =2
Graduation = 2ml
For cylinder C-
10-5 = 5
Number of spaces= 5
∴5/5 = 1
Graduation = 1ml
Hence , Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
To learn more about graduated cylinders refer-https://brainly.com/question/26216613
#SPJ9
Your question is incomplete. Please find the missing image below.
Given the balanced chemical equation: 2 H2 + O2 → 2 H20. Choose
the description below that most accurately describes the reaction.
O 2 moles of hydrogen react with 1 mole of oxygen to form 2 moles of water
O 2 moles of water react with 1 mole of hydrogen to form 2 moles of oxygen
O equal moles of hydrogen and oxygen react to form an equal amount of
water
O 4 moles of hydrogen react with 2 moles of oxygen to form 4 moles of water
Answers
Answer:
The answer is
2 moles of hydrogen react with 1 mole of oxygen to form 2 moles of water
use the drop‑down menus to label the statements as either true of false. sodium hydroxide can cause severe damage to skin and eyes.
Answers
The statement "Sodium hydroxide can cause severe damage to skin and eyes" is true. Sodium hydroxide is a highly corrosive substance and can cause burns, irritation, and damage when it comes into contact with the skin or eyes.
Sodium hydroxide is a highly caustic compound that can cause significant damage to the skin and eyes upon contact. The severity of the damage depends on the concentration of the sodium hydroxide solution, the duration of exposure, and the amount of skin or eye tissue affected. Sodium hydroxide reacts with the fats and oils in the skin and eyes, leading to the formation of soap-like substances called "salts of fatty acids." These salts can cause significant tissue damage, leading to pain, redness, swelling, and blistering.
Learn more about Sodium hydroxide: https://brainly.com/question/30460434
#SPJ11
Which of these describes “Al”?
Answers
Answer:
The aluminium cation Al3+ which is small and highly charged; and is polarizing and bonds aluminium forms tend towards covalency.
Explanation:
Marie anne lavoisier drew illustrations of the equipment she and her husband used in their experiments. Why was this important?.
Answers
Marie Anne Lavoisier realized how crucial it was to create drawings of the tools she and her husband employed in their research. In order to impart knowledge, this is crucial.
It is widely acknowledged that Lavoisier's major contributions to chemistry came mostly from his transformation of the field from a qualitative to a quantitative one. The discovery of the function of oxygen in combustion is what made Lavoisier famous. He challenged the phlogiston idea and identified and called the elements oxygen and hydrogen (1778). Lavoisier contributed to the development of the metric system, created the first comprehensive list of elements, and reformed chemical nomenclature. In addition to discovering that matter's mass never changes despite its form or shape, he also anticipated the creation of silicon in 1787. Lavoisier served as the general manager of the Ferme and was a key member of several aristocratic councils
Learn more about Lavoisier here:
https://brainly.com/question/11833531
#SPJ4
Sugar in water makes a solution, but sand in water does not.
What is the same about sugar in water and sand in water?
A.
They are both mixtures with two parts.
B.
They both have solids that settle to the bottom.
C.
They are both solids.
D.
They both have parts that are evenly mixed.
Answers
Answer:
B
Explanation:
because when you put sugar in water you actually have to stir it in the water or it will just seat at the bottom of the water it also the same for sand except whener you mix the sand or not it will not dissolve unlike the sugar.
Compare and Contrast Bohr’s Atomic Theory and Quantum Atomic Theory.
Answers
Answer:
i think it is right..

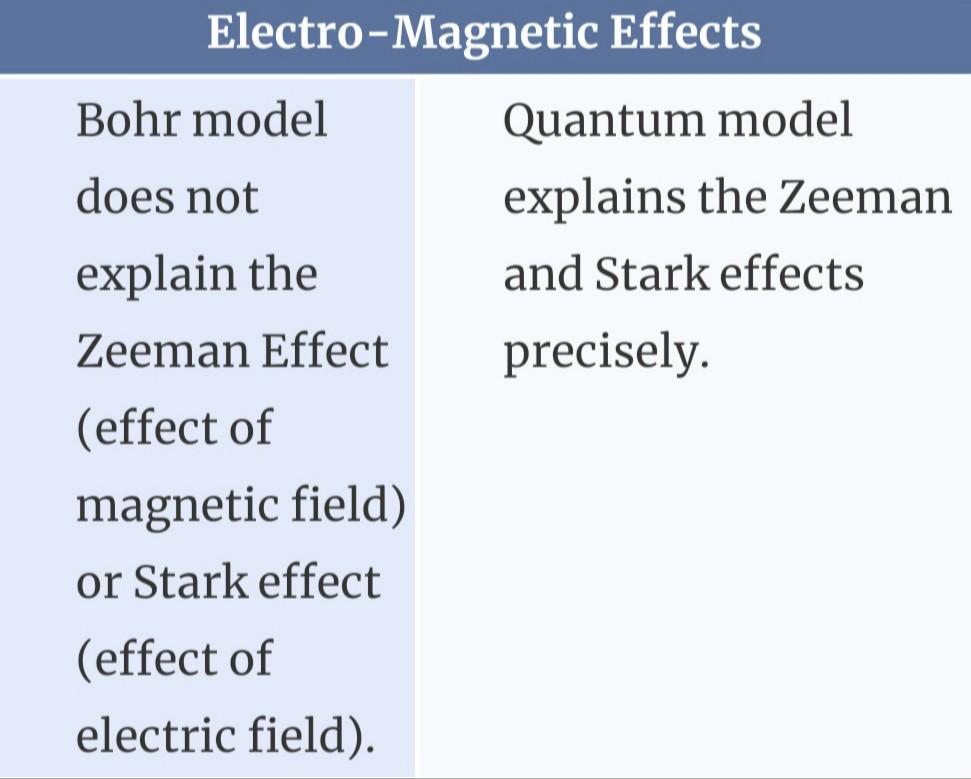
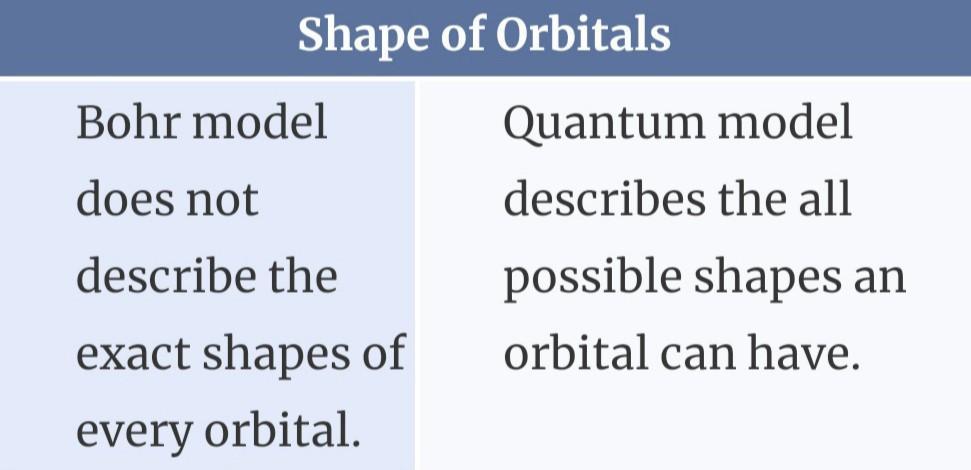
The primary distinction between both the Bohr model as well as the quantum atomic theory would be that the former describes the behavior of a particle as just a particle, while the latter describes its dual nature as a wave and even a particle.
What is Bohr model?To explain what electrons could maintain stable orbits all around the nucleus, Bohr suggested its quantized shell model of the atom.
What is quantum atomic theory ?According to quantum theory, energy exists in discontinuous packages known as quanta.
The electron was viewed as a particle with definite circular orbits in the Bohr Model. The electron gets mathematically handled as just a vibration in the quantum theory model. The electron possesses both particle- and wave-like characteristics.
To know more about quantum theory model and Bohr Model.
https://brainly.com/question/3964366
#SPJ2
Clear selection
Which country shares the Hueco Bolson aquifer with the United States?
a) Canada.
b) Mexico.
c) Florida
d) New Mexico
12 points
Answers
Answer:
b. mexico
Explanation:
The Hueco Bolson aquifer is a binational aquifer shared by the United States of America (USA) and Mexico that is strongly interconnected with the transboundary river, Rio Grande/Rio Bravo. Limited recharge, increasing urbanization, and intensified agriculture have resulted in the over-drafting of groundwater
A chemist has 2. 0 mol of methanol (CH3OH). The molar mass of methanol is 32. 0 g/mol. What is the mass, in grams, of the sample? 16 grams 30 grams 32 grams 64 grams.
Answers
The mass, in grams, of the sample of methanol (CH₃OH) is 64 grams.
How we calculate mass from moles?Mass of any substance can be calculated by using moles as:
n = W/M, where
W = required mass
M = molar mass
In the question that:
Moles of methanol = 2mole
Molar mass of methanol = 32g/mole
On putting these values in the above equation, we get
W = n × M
W = 2mole × 32g/mole = 64g
Hence, 64 grams is the mass of the sample.
To know more about moles, visit the below link:
https://brainly.com/question/15374113
Where dose the energy that drives the rock cycle come from
Answers
Answer:
It comes from the sun.
Calculate the pH and the pOH of an aqueous solution that is 0.045 M in HCl(aq) and 0.095 M in HBr(aq) at 25 °C. pH = pОН: =
Answers
The pH and the pOH of an aqueous solution that is 0.045 M in HCl(aq) and 0.095 M in HBr(aq) at 25 °C is 1.35, and 12.98 respectively.
To calculate the pH and pOH of the solution, we need to use the concentration of the acidic solutions and the dissociation constants of HCl and HBr.
First, calculate the pH:
For HCl (aq):
[HCl] = 0.045 M
HCl is a strong acid and dissociates completely in water, so the concentration of H⁺ ions is equal to the concentration of HCl:
[H⁺] = 0.045 M
Taking the negative logarithm (base 10) of the H⁺ concentration gives us the pH:
pH = -log10(0.045)
pH = 1.35
Now, let's calculate the pOH:
For HBr(aq):
[HBr] = 0.095 M
HBr is also a strong acid, and its dissociation is similar to HCl. The concentration of H⁺ ions is equal to the concentration of HBr:
[H⁺] = 0.095 M
Again, taking the negative logarithm (base 10) of the H⁺ concentration gives us the pH:
pH = -log10(0.095)
pH = 1.02
Since pH + pOH = 14 (at 25 °C), we can calculate the pOH:
pOH = 14 - pH
pOH = 14 - 1.02
pOH = 12.98
Therefore, the pH of the solution is approximately 1.35, and the pOH is approximately 12.98.
To know more about aqueous solution here
https://brainly.com/question/1382478
#SPJ4
What is the [H+] if the pH of a
solution is 2.0?
[ ? ]
× 10¹²]
X
[H+] =
Answers
The hydrogen ion concentration, [H⁺], given that the pH is 2.0 is 1×10⁻² M
How do I determine the hydrogen ion concentration, [H⁺]?We know that the pH of a solution is given by the following formula:
pH = -Log [H⁺]
Where
[H⁺] is the hydrogen ion concentrationWith that above formula, the hydrogen ion concentration, [H⁺] of the solution can be obtained as follow:
pH of solution = 2Hydrogen ion concentration [H⁺] = ?pH = -Log [H⁺]
2 = -Log [H⁺]
Multiply through by -1
-2 = Log [H⁺]
Take the anti-log of -2
[H⁺] = Anti-log -2
[H⁺] = 1×10⁻² M
Thus, from the above calculation, we can conclude that the hydrogen ion concentration, [H⁺] is 1×10⁻² M
Learn more about hydrogen ion concentration:
https://brainly.com/question/28595956
#SPJ1
An electrolytic cell is A. is cell in which reactant are continuously supplied to the cell B. a battery C. a cell in which an electric current drives a no spontaneous reaction D. a cell in which of the cell reaction is spontaneous
Answers
An electrolytic cell is a cell in which an electric current drives a no spontaneous reaction.
What is electrolytic cell?
Any apparatus in which excess electricity is changed into chemical energy or conversely is an electrolytic cell. Such a cell normally includes two electrodes, which can be metal or electrical conductors, kept apart from one another and in interface with an electrolyte (q.v. ), which is commonly an ionic compound that has been dissolved or fused.
For instance, water can be electrolyzed to create gaseous oxygen and gaseous hydrogen with the use of an electrolytic cell.
Electrical energy is transformed into chemical energy in an electrolytic cell. Electricity is generated in this situation as a result of a spontaneous redox reaction. Electricity must be provided to start the redox reaction because it is not spontaneous.
Therefore, Option C is correct.
To learn more about electrolytic cell
Here: https://brainly.com/question/19854746
#SPJ4
How many Fe(ii) ions are there in 20.0 g of FeSO4 (molar mass=151.9 g/mol) ? Avogadro number=6.0225x 10^23
Answers
Fe(ii) ions are there in 20.0 g of FeSO4 (molar mass=151.9 g/mol) ? Avogadro number=6.022× 10²³ is 0.794× 10²³
Mole concept is used for finding out the no of molecules present in a given sample.
Given,
Given mass =20g
Molar mass = 151.9/mol
Avogadro number=6.022× 10²³
We know,
Number of moles= Given mass / Molar mass
Number of moles= 20 / 151.9
Number of moles= 0.132 moles
According to the equation,
FeSO₄ -----> Fe⁺² + SO₄⁻²
Now,
Number of molecules of FeSO₄ = 0.132 × 6.022 × 10²³
Number of molecules of FeSO₄ = 0.794× 10²³
It can be seen from equation,
No. of molecules of FeSO₄ = no. of ions of Fe⁺² = no of ions of SO₄⁻²
No. of ions of Fe⁺² = 0.794× 10²³
Hence, No. of ions of Fe⁺² is 0.794× 10²³.
Learn more about Mole concept here, brainly.com/question/20668126
#SPJ4
Answer: The correct answer (not rounded) would be 7.93 × 1022 iron(II) ions
Hope that helps:)
Explain how the structure of ammonium lauryl sulfate, as described in parts E and F, produces the properties identified in part C. Write a short paragraph or two.
Answers
The structure of ammonium lauryl sulfate consists of 12 carbon atoms, 29 hydrogen atoms, 1 nitrogen atom, 4 oxygen atom and 1 sulfur atom.
Structure of ammonium lauryl sulfateThe molecular formula of ammonium lauryl sulfate is written as;
C12H29NO4S
The structure of ammonium lauryl sulfate consists of 12 carbon atoms, 29 hydrogen atoms, 1 nitrogen atom, 4 oxygen atom and 1 sulfur atom.
Properties of ammonium lauryl sulfateIt has a molar mass of 283.43 g/molIt has a density of 1.02 g/cm³It is light yellow viscous fluid, and it can sink in water due to its density.Learn more about ammonium lauryl sulfate here: https://brainly.com/question/27125444
#SPJ1
21. how many half-lives are required for the concentration of reactant to decrease to 25% of its original
Answers
Hence, it takes two half-lives for a reactant's concentration to drop to 25% of its initial value.
What does chemistry's definition of concentration entail?The amount of solute contained in a specific amount of solution is how concentrated a material is. The number of moles of solute in 1 L of solution, or molarity, is the standard unit of measurement for concentrations.
What is concentration, and what is an example of it?You can determine how much solute has dissolved in the solvent by looking at the concentration of the solution. One teaspoon of salt per two cups of water, for instance, would be the concentration.
To know more about Concentration visit:
https://brainly.com/question/10725862
#SPJ1
Potassium (K) and copper (Cu) are both in period 4. They both have _____.
four electron shells
similar properties
the same number of electrons
the same number of protons
Answers
Answer:
Similar properties
Explanation:
Potassium (K) has atomic number 19 which means its electron configuration (pattern of electrons in it's energy levels (electron shells)) is 2·8·8·1 while Copper (Cu) has atomic number 29 which means its electron configuration is 2·8·8·8·3 hence Potassium has 4 electron shells while Copper has 5
Potassium has 19 protons (from its atomic number) while Copper has 29 protons
The number of protons and electrons is usually the same (in an atom) (that's why atoms don't naturally have charges i.e they're usually neutral) so Potassium has about 19 electrons while Copper has about 29 electrons.
So similar properties automatically becomes your answer cuz the rest are wrong.
All objects that have
___________
have
___________
energy
Answers
Answer:
Potential and Kinetic energy
Explanation:
A reaction occurs between a piece of lithium metal and magnesium sulfate. What type of reaction is this?
Answers
The reaction between lithium metal and magnesium sulfate is a single displacement reaction, also known as a displacement or replacement reaction.
In this reaction, lithium metal (Li) reacts with magnesium sulfate (MgSO4) to form lithium sulfate (Li2SO4) and magnesium metal (Mg). The general equation for this reaction can be represented as:
2Li(s) + MgSO4(aq) -> Li2SO4(aq) + Mg(s)
In a single displacement reaction, one element displaces another element in a compound. In this case, lithium, being more reactive than magnesium, displaces magnesium from magnesium sulfate. The lithium atoms bond with the sulfate ions, forming lithium sulfate, while magnesium atoms are released as elemental magnesium.
Therefore, the reaction between lithium metal and magnesium sulfate is a single displacement reaction.
Learn more about displacement reaction Visit : brainly.in/question/2905135
#SPJ11
a solid sample of copper is an excellent fondue to if electric current which type of chemical bonds are in the sample
Answers
If a solid sample of copper is an excellent conductor of electric current, it is likely due to the presence of metallic bonds in the sample.
What is meant by good conductor?Good conductors are the materials which offer very low resistance to the flow of electric current.
Solid copper is a metal, and metals have metallic bonds. Metallic bonds are a type of chemical bond that occur between atoms of metallic elements. In a metallic bond, valence electrons of the metal atoms are delocalized and shared among all the atoms in metallic solid, creating "sea" of electrons that are free to move throughout the structure.
This gives metals their unique properties such as high electrical conductivity and ductility. So, if solid sample of copper is an excellent conductor of electric current, it is likely due to the presence of metallic bonds in sample.
To know more about conductor, refer
https://brainly.com/question/492289
#SPJ1
Note: The question given on the portal is incomplete. Here is the complete question.
Question: A solid sample of copper is an excellent conductor of electric current . Which type of chemical bonds are in the sample?
why does water boil at less than 100 drgrees celsius in boulder colorado
Answers
Explanation:
Because boiling point of water is not 100 degrees Celsius but it depends on atmospheric pressure. Liquid boils at temperature when partial pressure of liquid becomes equal to atmospheric pressure.
a sample of gas occupies a volume of 60.7 ml . as it expands, it does 138.1 j of work on its surroundings at a constant pressure of 783 torr . what is the final volume of the gas?
Answers
relationship between work pressure and volume can be stated by this formula;
W=PΔV
as we know gas is expanding so it means the work is done by the system
which is shown by a negative sign
W= 138.1 j x 1 L atm / 101.3 j
= - 1.36 L atm
let's convert torr into atm
P = 783 torr X ( 1 atm/ 760 torr)
= 1.03 atm
W = -PΔV
- 1.36 L atm = - 1.03 atm x ΔV
ΔV = 1.36 L atm / 1.03 atm
= 1.32 L
ΔV = \(V_{2}\) - \(V_{1}\)
= \(V_{2}\) - 0.0607 L
\(V_{2}\) = 1.32 L + 0.0607 L
= 1.38 L
the final volume of gas is 1.38 L
learn more work about here;
https://brainly.com/question/25573309
#SPJ4
50 mL graduated cylinder contains 25.0 mL of water. A 142.5040 g piece of osmium is placed in the graduated cylinder and the water level rises to 31.3 mL. What is the density of the piece of osmium?
Answers
Answer:
22.6g/mL
Explanation:
density = mass / volume
given mass of object : 142.5040
volume of object : not given however we are told that when placed inside of a cylinder with 25 mL of water the water level rises to 31.3 mL
31.3 - 25 = 6.3
So the water level rose by 6.3 mL meaning that the object has a volume of 6.3 mL
Now to find the density.
Recall that density = mass / volume
mass = 142.5040 g and volume = 6.3 mL
so density = 142.5040g / 6.3 mL = 22.6 g / ml
