Answers
Answer:
A. Plants
Explanation:
Plants are producers, which make their own food. Other organisms, such as rodents, birds, and wild cats, are consumers, which receive their energy from plants or other consumers.
Related Questions
calculate the number of moles for the quanity 8.06 x 1021 atoms of Pt
Answers
The number of moles for the quanity 8.06 x\(10_{21\) atoms of Pt is approximately 2.61 grams.
To calculate the number of moles for a given quantity of atoms, we can use Avogadro's number and the molar mass of the element. Avogadro's number is 6.022 x 10²³ atoms/mol.
In this case, you have 8.06 x 10²¹ atoms of Pt. To find the number of moles, divide this quantity by Avogadro's number:
8.06 x 10²¹ atoms Pt / 6.022 x 10²³ atoms/mol = 0.0134 mol Pt
So, there are approximately 0.0134 moles of Pt in 8.06 x 10²¹ atoms of Pt.
The molar mass of Pt (platinum) is 195.08 g/mol. To convert the number of moles to grams, multiply the number of moles by the molar mass:
0.0134 mol Pt x 195.08 g/mol = 2.61 g Pt
Therefore, there are approximately 2.61 grams of Pt in 8.06 x10²¹ atoms of Pt.
In summary, the number of moles for the quantity 8.06 x 10²¹ atoms of Pt is approximately 0.0134 moles. This is equivalent to approximately 2.61 grams of Pt. Remember to use Avogadro's number and the molar mass to perform these calculations accurately.
Know more about moles here:
https://brainly.com/question/29367909
#SPJ8
Which of the following are found outside the nucleus?
Select 1 correct answer(s)
neutron
proton
electron
Answers
electrons
Reason:
neutrons and protons are inside the nucleus
Answer:
The electron e^- is found outside the nucleus hope this helps. the proton and the neutron is found inside the nucleus leaving only the electron
How many atoms are in a 3.5 mole sample of the element lithium?
Answers
Answer:
2.1 x 10^24
Explanation:
The number of atoms in a mole will always be the same. The fact that it's lithium is completely irrelevant.
One mole of anything is 6.022 x 10^23 atoms
So if we need to find the number of atoms in 3.5 mol we just need to multiply Avogadro's number by 3.5
3.5 x 6.022 x 10^23
= 21.077 x 10^23
To make it proper scientific notation the first number can't be greater than 10 so let's move the decimal place and increase the exponent
2.1077 x 10^24
Remove the numbers that aren't significant figures
2.1 x 10^24
Boom there's your answer
Fsdfsfsdfsdfsdfsdfsdfsdf
Answers
Answer:
No
Explanation:
No
NH3 is a weak alkali that does not dissociate fully into its solution. Which of the following is true about NH3?
A. It has a very low pH.
B. It's dissociation is a reversible reaction.
C. It has a high H+ concentration.
D. It will release all of its OH- ions.
Answers
Answer:
Answer:
B. It's dissociation is a reversible reaction
Explanation:
NH3 is a weak alkali that does not dissociate fully into its solution. Only parts of the ammonia takes part in the dissociation process.
NH3 + H20 —> NH4+ + OH-
This dissociation is reversible which means the reactants can be formed from the product gotten from the dissociation
It has a high pH due to its basic nature. It also has a Low concentration of H+ ions and not all the OH- ions are released.
PLZZZ HELPP!!!
the cost of an energy drink is $2.00. If you drink an energy drink every day for three years, approximately how much money will be spent? Explain how you solved the problem using conversion factors
Answers
Answer:
1,095 x 2= $2,190
year= 365 days
day= 1 day
1 day = $2.00
i knew that 1 year was 365 I so i did 365x3 to get 1,095 x 2 to get how much i would spent in 3 years to get 2,190
Explanation:
cool
Can y’all please help me with these 2? I had surgery and missed this entire unit and it’s due Monday
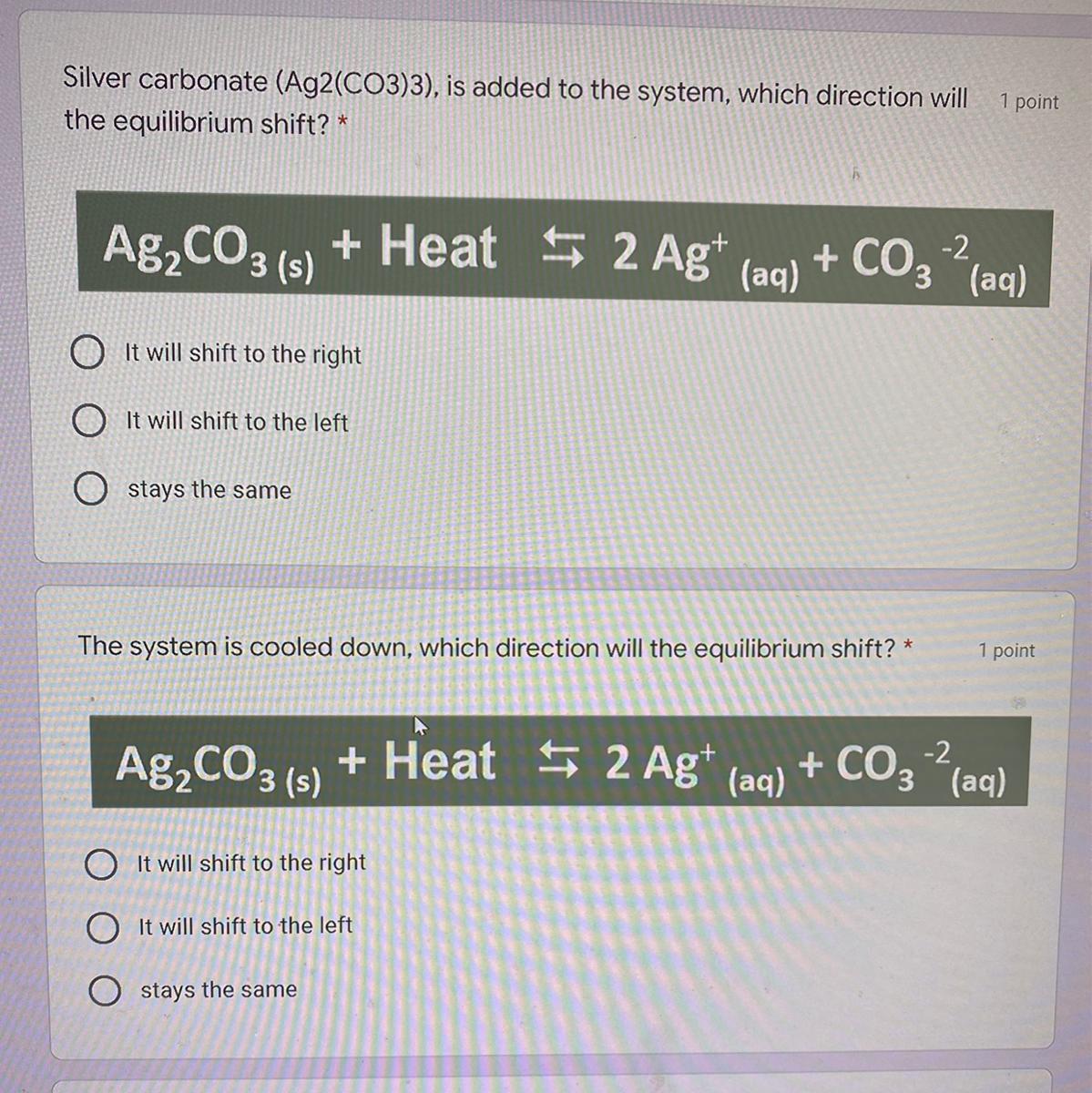
Answers
Answer :
1. It will shift left ( as heat is added)
2. It will shift right ( as it cooled down)
Explanation :
For an exothermic reaction, heat is a product. Therefore, increasing the temperature will shift the equilibrium to the left, while decreasing the temperature will shift the equilibrium to the right.
how many elements are in Li2SO4?how many elements are in li2s 04 how many elements are in li2s 04
Answers
Answer:
3
Explanation:
the three elements involved in this compound are Li, S, O.
lithium, sulfur, and oxygen. Which create the ionic compound, "Lithium Sulfate."
7 atoms total, since there are two lithium, four oxygen, and one sulfate atom. this is a white inorganic salt.
There are three different types of elements in LiSO₄ which are lithium (Li), sulfur (S), and oxygen (O).
What is an element?
An element is a pure substance made of only one kind of atom which all have the same number of protons in their nuclei.
The chemical element was first presented by Robert Boyle who defined it as "incapable of decomposition”. Boyle’s this definition lies admirably close to present-day theory.
When different kinds of elements undergo particular chemical reactions then atoms are rearranged into new compounds connected together by chemical bonds.
All other naturally occurring elements occur on the Earth as compounds or mixtures. Air is a mixture of the elements nitrogen (N₂), oxygen (O), and argon (Ar), though it does contain carbon dioxide and water.
Given compound, lithium sulfate is formed of LiSO₄ has elements lithium, sulfur, and oxygen.
Learn more about the chemical element, here:
https://brainly.com/question/9249660
#SPJ2
Identify the state(s) of matter that each property describes.
takes the shape of its container:
gas
liquid
solid
fills all available space:
gas
liquid
solid
maintains its shape:
gas
liquid
solid
can be poured:
gas
liquid
solid
is compressible:
gas
liquid
solid
has a fixed volume:
gas
liquid
solid
Answers
Answer:
1) takes the shape of its container ( liquid and gas).
2) fills all available space (gas)
3) maintains its shape ( solid)
4) can be poured ( liquid)
5) is compressible ( gas)
6) has a fixed volume ( liquid and solid)
Explanation:
Matter is simply defined as anything that has weight and occupies space. It exists in three states, namely: solid, liquid and gaseous states.
The properties of different states of matter includes:
SOLID STATE
--> It has a definite shape: The shape of a solid is fixed; it does not depend on the shape of other materials.
--> It has a definite volume: it occupies its own shape due to the force of cohesion among its molecules.
--> It is tightly packed: The molecular movements of particles are negligible.
LIQUID STATE
--> liquid has a defined volume.
--> it has no definite shape: There is no specific shape of a liquid. It occupies any available space. It's shape depends on the shape of the container into which it is poured.
GASEOUS STATE
--> it has no fixed shape: Due to the distance in the molecules of gas, the gaseous state has no shape. It occupies the shape of its container.
--> It has no fixed volume: it occupies the shape of any container that is closed.
--> It is highly compressible: The particles of gas are far off from one another and there is room for collision.
14. The illustration below shows two atoms of a fictitious element (M) forming a diatomic
molecule. What type of bonding occurs between these two atoms?
A. Covalent
B. Hydrogen
C. lonic
D. Polar

Answers
Covalent bonding involving covalent bonds is depicted between these two atoms as they form diatomic molecule.
What is a covalent bond?Covalent bond is defined as a type of bond which is formed by the mutual sharing of electrons to form electron pairs between the two atoms.These electron pairs are called as bonding pairs or shared pair of electrons.
Due to the sharing of valence electrons , the atoms are able to achieve a stable electronic configuration . Covalent bonding involves many types of interactions like σ bonding,π bonding ,metal-to-metal bonding ,etc.
Sigma bonds are the strongest covalent bonds while the pi bonds are weaker covalent bonds .Covalent bonds are affected by electronegativities of the atoms present in the molecules.Compounds having covalent bonds have lower melting points as compared to those with ionic bonds.
Learn more about covalent bond,here:
https://brainly.com/question/19382448
#SPJ2
Please look at attached image !!!

Answers
Ideal gas law is valid only for ideal gas not for vanderwaal gas. Therefore 26.55g of iron is needed to completely consume 18.5L chlorine gas at 25 degree Celsius and 1 atm pressure.
What is ideal gas equation?Ideal gas equation is the mathematical expression that relates pressure volume and temperature.
Mathematically the relation between Pressure, volume and temperature can be given as
PV=nRT
where,
P = pressure of chlorine gas =1 atm
V= volume of chlorine gas =18.5L
n =number of moles of chlorine gas
T =temperature of chlorine gas =298K
R = Gas constant = 0.0821 L.atm/K.mol
1 atm×18.5L= 0.0821×n×298K
1 atm×18.5L=n×24.4
n=0.75mol
mass of chlorine=0.75mol×35.453 g/mol
=26.55g
Therefore 26.55g of iron is needed to completely consume 18.5L chlorine gas at 25 degree Celsius and 1 atm pressure.
To learn more about ideal gas equation, here:
https://brainly.com/question/14826347
#SPJ1
Shown above is the phase diagram for water as it is heated. Which section represents the phase of water with the highest kinetic energy?
Answers
The section that represents the phase of water with the highest kinetic energy is the gas phase or vapor phase.
Gas phase or vapor phase section is above the boiling point curve, which separates the liquid and gas phases. At this point, the temperature is at or above 100°C (at standard atmospheric pressure), and the kinetic energy of the water molecules is sufficient to overcome the intermolecular forces holding them in the liquid phase and escape into the gas phase. The gas phase has the highest kinetic energy because the water molecules in this phase are more widely separated and move more rapidly than in the liquid or solid phases. The gas phase is also characterized by the highest entropy or disorder, as the molecules are free to move in any direction and occupy a large volume. The section that represents the phase of water with the highest kinetic energy is gas phase or vapor phase.
for more questions on water
https://brainly.com/question/19491767
#SPJ11
What are the products of the chemical reaction shown?
CH4+2O2 --> CO2 + 2H2O
Question 10 options:
CH4 and O2
O2 and H2O
CO2 and H2O
CH4 and CO2
Answers
Answer:
CO2 AND H20 I THINK IM 99 PERCENT SURE
How would each of the following procedural errors affect the value obtained for the
molar volume of hydrogen gas? Explain your reasoning.
(a) Some bubbles of hydrogen gas remained clinging to the sides of the tube.
(b) Some magnesium metal was left un-reacted at the end of the experiment.
(c) 7 mL of the HC1 were used instead of 5 mL.
Answers
Molar volume of hydrogen gas is because 'some bubbles of hydrogen gas remained clinging to the sides of the tube'
The volume occupied by 1 mole of the molecules of a gas at STP i.e. standard temperature and pressure
Here if liquid is not vaporized completely then bubbles of hydrogen gas remained clinging to the sides of the tube and then the condensed vapor in the flask contains the air which is initially occupied before the liquid is heated and when calculating the molar mass of the vapor the moles of air which are initially present are not excluded, so that the molar mass of the vapor would be increase in large value
Know more about molar volume
https://brainly.com/question/28634547
#SPJ1
Oxygen is not the simplest element.
True or false
Answers
Answer:
false, Hydrogen is the simplist
Hydrogen is the simplest element; its atom consists of only one proton and one electron
How do I balance this?
_CuC12 + _NaNO3 → _CU(NO3)2 + _ NaC1
Answers
\(CuCl_2+2NaNO_3 \rightarrow Cu(NO_3)_2 + 2NaCl\)
Which of these is a function of all cells ?
A. To produce oxygen through photosynthesis
B. To combine with other cells to form tissues
C. To subdivide to form cells for sexual reproduction
D. To transfer genetic information from one generation to the next
Answers
Answer:
a because it help in protecting disease
When 2 moles of Fe(s) react with Cl2(g) to form FeCl3(s) according to the following equation, 799 kJ of energy are evolved.
2Fe(s) + 3Cl2(g) —----2FeCl3(s)
1. Is this reaction endothermic or exothermic?
2. What is the value of q?
Answers
Answer:
1. Exothermic.
2. -1598 kJ.
Explanation:
Hello!
1. In this case, according to the reaction, we can infer that 799 kJ of energy are evolved (given off, released) it means that the enthalpy of reaction is negative as the reactants have more energy than the products; which means this is an exothermic reaction.
2. Here, as we know that the enthalpy of reaction is -799 kJ/mol, we can compute the q-value as shown below, considering the reacted 2 moles of solid iron:
\(q=2mol*-799 kJ/mol\\\\q=-1598kJ\)
Which means that 1598 kJ of energy are evolved when 2 moles of solid iron react.
Best regards!
Americium-241 is widely used in smoke detectors. The radiation released by this element ionizes particles that are then detected by a charged-particle collector. The half-life of 241Am is 432 years, and it decays by emitting alpha particles. How many alpha particles are emitted each second by a 5.54-g sample of 241Am
Answers
Answer:
Explanation:
Half life T is 432 years .
432 years = 432 x 365 x 24 x 60 x 60 s
= 1.36 x 10¹⁰ s .
No of atoms in 5.54 g of sample of Am N= 6.02 x 10²³ x 5.54 / 241
N = 138.38 x 10²⁰ atoms .
disintegration constant λ = .693 / half life
λ = .693 / 1.36 x 10¹⁰ s .
= .51 x 10⁻¹⁰ .
dN / dt = λN
= .51 x 10⁻¹⁰ x 138.38 x 10²⁰
= 70.57 x 10¹⁰ per second.
No of particles emitted = no of disintegration per second
No of particles emitted = 70.57 x 10¹⁰ per second.
What would the products be for the reaction between Na3PO4 + MgSO4?
Answers
MgSO4 + Na3PO4 = Na2SO4 + Mg3(PO4)2
Answer: The products of Na3PO4 + MgSO4 are Na2SO4 + Mg3(PO4)2
Explanation:
What is the formula for tin(IV) sulfide?
A. Sn4S
B. SnS2
C. Sns
D. SnS4
Answers
Answer:
\(SnS_{2}\)
Explanation:
The formula for tin(IV) sulfide is SnS\(_{2}\)
A piece of an unknown metal with mass 23.8g is heated to 100.0 degrees Celsius and dropped into 50.0
cm³ of water at 24.0 degrees Celsius. The final temperature of the system is 32.5 degrees Celsius. What is the specific heat of the metal?
Answers
The specific heat of the metal is 1.1106 J/g°C
Specific heat is the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree
Here given data is
Mass of unknown metal = 23.8g
Temprature = 100°C
Mass of water = 50.0cm³ = 50 g
Temprature of water = 24.0°C
Final temprature of the system = 32.5°C
We have to find specific heat = ?
So first we determine the heat gain by water
Specific heat of water is 4.18 J/g°C
Q = mcΔT
Q = 50 g×8.5°C×4.18 J/g°C
Q = 1776.5 Joules
Then we determine the total heat lost by the unknown metal
Taking the specific heat f the metal to be x
Heat = 23.8g×67.5°C×x
1776.5 Joules = 23.8g×67.5°C×x
1776.5 Joules = 1606.5 J
x = 1606.5 J/1776.5 Joule
x = 1.1106 J/g°C
Specific heat of the metal is 1.1106 J/g°C
Know more about metal
https://brainly.com/question/15188305
#SPJ9
If 325 J of work is done by a system at a pressure of 1.0 atm and 298 K, what is the change in the volume of
the system? Give the answer in L.
Answers
The change in volume of the system if 325 J of work is done by a system is -3.21L.
How to calculate volume?To calculate how much work a gas has done (or has done to it) against a constant external pressure, the following formula is used;
W = -P × ∆V
Where;
W = work doneP = pressure∆V = change in volumeAccording to this question, if 325 J of work is done by a system at a pressure of 1.0 atm and 298 K. The change in volume can be calculated as follows:
325J is equivalent to 325/101.325 = 3.21L/atm
3.21 = -1 × ∆V
3.21 = -∆V
∆V = -3.21L
Learn more about volume at: https://brainly.com/question/24189159
#SPJ1
ASAP PLS
A 18.7 g piece of aluminum (which has a heat capacity of 0.89 JPC-g) is
heated to 82.4°C and dropped into a calorimeter containing water
(specific heat capacity of water is 4.18 J/gºC) initially at 22.3°C. The final
temperature of the water is 24.3°C. Ignoring significant figures, calculate
the mass of water in the calorimeter. *

Answers
Answer:
think I did this before and its V
Trans-4-hexen-3-ol can be synthesized starting from acetaldehyde. One of the key reagents is ethyl grignard.
1. Synthesize ethyl grignard from acetaldehyde in the steps below using the reagents provided.
2. Synthesize (trans)-4-hexen-3-ol from acetaldehyde.
Answers
find the given attachment

4. Which formula represents the binary molecular compound dinitrogen tetroxide?
O N₂0
ON₂04
O NO4
O N₂02
Answers
N₂0₄ is the formula represents the binary molecular compound dinitrogen tetroxide. Therefore, option B is correct.
What is the binary molecular compound ?Binary molecular compounds are made up of exactly two nonmetal elements. HF, NO2, and P2O5 are a few examples. It is extremely simple to name binary molecular compounds. The first element is given its element name, followed by ide; the second element is given its root (hydr, bor, carb, ox, fluor, etc.).
In N₂0₄ molecule there are two nitrogen atoms at the suffix so, it is binary and in oxygen atom its suffix is 4 so, it is tetroxide. Therefore, N₂0₄ is the formula represents the binary molecular compound dinitrogen tetroxide.
Thus, option B is correct.
To learn more about the binary molecular compound, follow the link;
https://brainly.com/question/7960132
#SPJ1
how many moles of F are in 708.5g of F4C?
Answers
The number of mole of F present in 708.5 grams of F₄C is 32.2 moles
How do i determine the number of mole of F present 08.5 grams of F₄C?First, we shall determine the mole in 708.5 grams of F₄C. Details below:
Mass of F₄C = 708.5 grams Molar mass of F₄C = 88 g/mol Mole of F₄C =?Mole = mass / molar mass
Mole of F₄C = 708.5 / 88
Mole of F₄C = 8.05 moles
Finally, we shall determine the number of mole of F in 708.5 grams (i.e 8.05 moles) of F₄C. Details below:
From the chemical formula of F₄C,
1 mole of F₄C contains 4 moles of F.
Therefore,
8.05 moles of F₄C will contain = (8.05 mole × 4 mole) / 1 mole = 32.2 moles of F.
Thus, we can conclude from the above calculation that the number of mole of F present is 32.2 moles
Learn more about mole composition:
https://brainly.com/question/21323029
#SPJ1
Which element is not found in the skeleton of amino acids?
Answers
Answer:
Serine. Serine is non-essential amino acid supplied from food or synthesized by the body from a number of metabolites, including glycine. Serine is found in soybeans, nuts (especially peanuts, almonds, and walnuts), eggs, chickpeas, lentils, meat, and fish (especially shellfish).
Explanation:
vodka from the european union must be at least 37.5% alcohol by volume or 75 proof. The density of water is 1.00 g mL^-1 and the density of ethanol is 0.789 g mL ^-1
What is the mole fraction of alcohol in vodka?
What is the molarity of alcohol in vodka?

Answers
Ininihi my bad
Answer:
To calculate the mole fraction of alcohol in vodka, we need to know the volume fraction of alcohol in vodka, since ethanol and water have different densities.
75 proof vodka means it contains 37.5% ethanol by volume. So for 100 mL of vodka, there are 37.5 mL of ethanol and 62.5 mL of water.
The mass of 37.5 mL of ethanol is:
37.5 mL * 0.789 g/mL = 29.5875 g
The mass of 62.5 mL of water is:
62.5 mL * 1.00 g/mL = 62.5 g
The total mass of 100 mL of vodka is:
29.5875 g + 62.5 g = 92.0875 g
The mole fraction of alcohol in vodka is:
moles of ethanol / (moles of ethanol + moles of water)
To calculate the moles of ethanol, we can divide the mass of ethanol by its molar mass:
moles of ethanol = 29.5875 g / 46.07 g/mol = 0.6412 mol
To calculate the moles of water, we can divide the mass of water by its molar mass:
moles of water = 62.5 g / 18.02 g/mol = 3.4739 mol
The mole fraction of alcohol in vodka is therefore:
0.6412 mol / (0.6412 mol + 3.4739 mol) = 0.155
To calculate the molarity of alcohol in vodka, we need to divide the moles of ethanol by the volume of the vodka in liters:
moles of ethanol = 0.6412 mol
volume of vodka = 100 mL = 0.1 L
molarity of ethanol = moles of ethanol / volume of vodka
molarity of ethanol = 0.6412 mol / 0.1 L = 6.412 M
Therefore, the molarity of alcohol in vodka is 6.412 M.
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale). If this is true, what determines the difference between a slate and a gneiss rock that both are formed from shale? What role does the parent rock play in determining the type of metamorphic rock that will be formed?
Answers
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale) is a true statement.
The parent rock, in this case shale, plays a significant role in determining the type of metamorphic rock that will be formed. The minerals and structure of the parent rock provide the starting material for the metamorphic rock, and the specific conditions under which the rock undergoes metamorphism determine the final characteristics of the metamorphic rock.What determines the difference between a slate and a gneiss rock that both are formed from shale?Slate, phyllite, schist, and gneiss are all types of metamorphic rocks that can be formed from shale, which is a sedimentary rock composed of clay and other fine-grained minerals. The specific type of metamorphic rock that is formed from shale depends on the conditions under which the shale undergoes metamorphism, including the temperature, pressure, and presence of fluids.
Slate is a fine-grained metamorphic rock with a uniform, flat surface and a layered structure. It is formed when shale undergoes low-grade metamorphism, which occurs at relatively low temperatures and pressures.
Therefore, Gneiss, on the other hand, is a medium- to coarse-grained metamorphic rock with a banded or wavy texture. It is formed when shale undergoes high-grade metamorphism, which occurs at higher temperatures and pressures.
Learn more about Metamorphic Rock from
https://brainly.com/question/1176274
#SPJ1