Answers
Answer: Solids and liquids don't suddenly change their volumes.
But gases do...they expand to fill whatever container you
put them in.
Michael's mystery substance is in the gaseous phase.
Explanation:
Related Questions
Which are the functions of the skin? Check all that apply.
helps control body temperature
moves parts of the body
serves as a barrier of protection
releases waste materials
produces vitamin A
gives the body support and shape
Answers
Answer:
A,C,D
Explanation:
I TOOK THE TEST
Please help find out code?

Answers
How does nitrogen in the atmosphere become beneficial to plants and eventually animals?
O Nitrogen must be mixed in the soil by earthworms,
O Nitrogen must be converted into a useful form by bacteria,
O Nitrogen must come in contact with decaying organic material,
O Nitrogen must come in contact with the ground through rainfall
Answers
Answer:
O Nitrogen must be converted into a useful form by bacteria.
This bacteria is called the nitrogen fixing bacteria such as the Rhizobium bacteria.
What is solar atomic?
Answers
Solar atomic refers to an atomic watch that is a type of radio-controlled wristwatch that tells time by monitoring the resonance frequency.
What is meant by solar atomic watch?An atomic watch is consistently serialized by receiving radio signals from atomic clocks. As a result, They exhibit the time correctly and do not need battery change to operate flawlessly. These special watches come in various styles, and sizes, and plan for men and women, making them appealing to everyone.
Because solar watches are always recharging their batteries, they reliably tell time with unparalleled correctness with many solar watches vastly exceeding their atomic timekeeping, quartz, mechanical, and automatic complement.
So we can conclude that atomic watches are the most error-free watches there are on the market.
Learn more about atomic here: https://brainly.com/question/6258301
#SPJ1
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
Given the reaction: Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
The reaction occurs more rapidly when a 10-gram sample of Mg is powdered rather than in one piece, because powdered
Mg has
1. less surface area
2. more surface area
3. a lower potential energy
4. a higher potential energy
Answers
How many grams are in 5.2 grams of K
Answers
If so, the answer should be 0.0052.
South Florida is surrounded by salt water. The need for fresh
drinking water has increased as the population has exploded. Only
10% of our water comes from surface waters, the other 90% is
pumped up from ground water sources. What is the name of this
ground water source?
lakes
sinkholes
O deltas
o aquifers
Answers
Answer:
Aquifer
Explanation:
Instead of snowcapped mountains that store water in advance of warmer temperatures, most of our drinking water comes from underground "mountains" of porous materials called aquifers which are replenished by rain. The Biscayne Aquifer is South Florida's lower east coast's primary source of fresh water.
The pressure inside a tire is measured as 28.0 . What is its pressure in ?
1 pound = 4.45 newtons
1 inch2 = 6.45 centimeters2
Express the answer to the correct number of significant figures.
The pressure is
Answers
The pressure inside the tire, converted to pascals, is approximately 8,555 N/m^2, or 8.56 × 10^3 Pa, using the appropriate number of significant figures.
To convert the pressure from pounds per square inch (psi) to pascals (Pa), we need to use the given conversion factors:
1 pound = 4.45 newtons
1 inch^2 = 6.45 centimeters^2 = (6.45/100)^2 square meters
First, let's convert psi to newtons per square inch (N/in^2):
28.0 psi * 4.45 N = 124.6 N/in^2
Now, let's convert newtons per square inch to pascals:
124.6 N/in^2 * ((6.45/100)^2) m^2 = 8,555.4125 N/m^2 (approximately)
To express the answer to the correct number of significant figures, we need to determine the number of significant figures in the given pressure value. Since the pressure is given as "28.0," it implies that there are three significant figures. Therefore, the pressure inside the tire, converted to pascals, is approximately 8,555 N/m^2, or 8.56 × 10^3 Pa, using the appropriate number of significant figures.
For more question on pressure
https://brainly.com/question/24719118
#SPJ8
5. What would be the temperature change if 12.5 g of water absorbed 35 J of heat?
Answers
Explanation:
its 399929939932929292
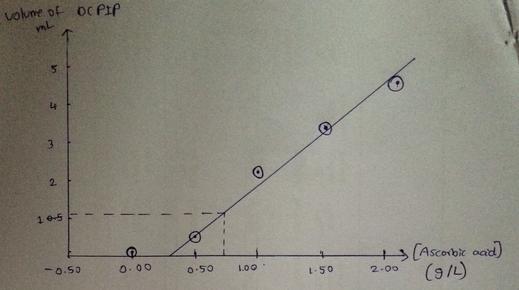
Based on the results of your titration, what volume of the sample would an adult need to consume to reach the recommended daily amount? 60 mg is the recommened daily amount of vitamin C.
Ascorbic acid Volume of DCPIP in Total Volume of DCPIP
concentration (g/L) final syringe (mL) used (mL)
0.00 2.18 mL 0.18 mL
0.50 3.1 mL 0.6 mL
1.00 4.6 mL 2.2 mL
1.50 5.56 mL 3.39 mL
2.00 6.37 mL 4.37 mL
Unknown Trial 1 3.21 mL 1.21 mL
Unknown Trial 2 3.11 mL 1.11 mL
Unknown Trial 3 3.15 mL 1.06 mL
Answers
Answer: 85.7 mL
Explanation:
Given the information from the question as plotted in the graph i will be uploading along side this answer,
Average of total volume of DCPIP used is
= (1.21 + 1.11 + 1.06)mL / 3
= 1.12 mL
and corresponding ( ascorbic acid ) is 0.70 g/L
Two parameter given as volume of DCPIP in final syringe and total volume of DCPIP are quite ambiguous
700mg ⇒ 1 L
THEREFORE volume that contains 60mg = (1000/700) × 60 = 85.7 mL
Pls give a detailed explanation about what are enzyme mutations
Answers
Answer:
Enzyme mutations can lead to serious or fatal human disorders and are the consequence of inherited abnormalities in the affected individual's DNA. The mutation may be at a specific position in an enzyme encoded by a mutated gene, just like a single abnormal amino acid residue.
Explanation:
It takes 4.37 J of heat to raise the temperature of Object A by 1oC, and 2.88 J to raise the temperature of Object B by 1oC. Suppose A and B are brought into contact. A is initially hotter. A is seen to cool down by 6.3oC . How would you calculate the rise in temperature of B? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols.
Answers
A heat capacity minus A temperature loss equals B heat capacity minus B temperature gain 9.56 °C
Object A is hotter than Object B, and since they are in direct contact, heat can be conducted between them. The conservation of energy principle states that energy cannot be created or destroyed. The energy that item A expends in this situation can only be transferred to object B.
To calculate the amount of energy transmitted, multiply the heat capacity by the degrees of temperature increase or drop, assuming that the mass of both items is the same. Below is the calculation:
Energy acquired by B = Energy lost by A
A heat capacity minus A temperature loss equals B heat capacity minus B temperature gain.
\(4.37J/degree C * 6.3 C= 2.88 J * TbTb= \frac{(4.37 J/ degree C * 6.3 C) }{ 2.88J}Tb= 9.56 degree C\)
Therefore ,A heat capacity minus A temperature loss equals B heat capacity minus B temperature gain 9.56 °C
learn more about heat capacity Refer:brainly.com/question/13368849
#SPJ4
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
Which functional groups are present in carbohydrates?
alkenes, alkynes, and phenyl groups
ethers, esters, and amides
carboxylic acids and amino groups
aldehydes, ketones, and hydroxyl groups
Answers
The functional groups are present in the carbohydrates is aldehydes , ketone , and hydroxyl groups.
Carbohydrates are the essential for living thing . they provide energy to living things. simple carbohydrates are : monosaccharides , disaccharides, oligosaccharides. Carbohydrates involves in fat metabolism. carbohydrates are also called as starch , simple sugars etc. carbohydrates found in foods . sugar is a source of carbohydrates. there are good carbohydrates which is high in nutrients moderate in calories. the bad carbohydrates are low in nutrients and high in calories.
Thus, The functional groups are present in the carbohydrates is aldehydes , ketone , and hydroxyl groups.
To learn more about carbohydrates here
https://brainly.com/question/14614055
#SPJ1
Calculate the pH of a buffer solution that contains 0.25 M benzoic acid (C 6H 5CO 2H) and 0.15M sodium benzoate (C
Answers
Answer:
\(pH=3.97\)
Explanation:
Hello,
In this case, for the calculation of the pH of the given buffer we need to use the Henderson-Hasselbach equation:
\(pH=pKa+log(\frac{[base]}{[acid]} )\)
Whereas the pKa for benzoic acid is 4.19, the concentration of the base is 0.15 M (sodium benzoate) and the concentration of the acid is 0.25 M (benzoic acid), therefore, the pH turns out:
\(pH=4.19+log(\frac{0.15M}{0.25M} )\\\\pH=3.97\)
Regards.
Homeostasis in a constantly changing environment is a process known as
Answers
How many molecules are in
5.657g H2SO4?
Answers
There are approximately 3.47 x 10²² molecules in 5.657g H₂SO₄.
To calculate the number of molecules in 5.657g H₂SO₄, we need to use the Avogadro's number and the molar mass of H₂SO₄.
The molar mass of H₂SO₄ is 98.079 g/mol.
We need to calculate the number of moles of H₂SO₄:
Number of moles = mass/molar mass
= 5.657g / 98.079 g/mol
= 0.05767 mol.
Then, we can use Avogadro's number, which is 6.022 x 10²³ molecules/mol, to find the number of molecules:
Number of molecules = number of moles x Avogadro's number
= 0.05767 mol x 6.022 x 10²³ molecules/mol
= 3.47 x 10²² molecules
To calculate the number of molecules in a given sample of a substance, you need to use the Avogadro's number, which is 6.022 x 10²³ molecules/mol. This means that one mole of a substance contains 6.022 x 10²³ molecules.
We are given the mass of H₂SO₄, which is 5.657 g. To calculate the number of molecules, we first need to determine the number of moles of H₂SO₄ in the given sample. The molar mass of H₂SO₄ is 98.08 g/mol. So, the number of moles of H₂SO₄ can be calculated as follows:
moles = mass / molar mass
moles = 5.657 g / 98.08 g/mol
moles = 0.0576 mol
Now, we can use the Avogadro's number to determine the number of molecules of H₂SO₄ in 0.0576 moles:
number of molecules = moles x Avogadro's number
number of molecules = 0.0576 mol x 6.022 x 10²³ molecules/mol
number of molecules = 3.47 x 10²² molecules
As a result, in 5.657 g of the material, there are roughly 3.47 x 1022 molecules of H₂SO₄.
To know more about the Molecules, here
https://brainly.com/question/11488454
#SPJ1
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
A secondary step in the process to produce ultra-pure silicon is to combine silicon tetrachloride with magnesium. How many grams of Si could be produced by reacting 2.00 kg of SiCl4 with excess Mg
Answers
The mass of silicon, Si produced from the reaction is 329.41 g
Balanced equationSiCl₄ + 2Mg —> 2MgCl₂ + Si
Molar mass of SiCl₄ = 28 + (35.5×4) = 170 g/mol
Mass of SiCl₄ from the balanced equation = 1 × 170 = 170 g
Molar mass of Si = 28 g/mol
Mass of Si from the balanced equation = 1 × 28 = 28 g
From the balanced equation above,
170 g of SiCl₄ reacted to produce 28 g of Si.
How to determine the mass of Si producedFrom the balanced equation above,
170 g of SiCl₄ reacted to produce 28 g of Si.
Therefore,
2 Kg (i.e 2000 g) of SiCl₄ will react to produce = (2000 × 28) / 170 = 329.41 g of Si
Thus, 329.41 g of Si were obtained from the reaction
Learn more about stoichiometry:
https://brainly.com/question/14735801
2. Enumerate four Reactive Oxygen Species (ROS)
Answers
Answer:
Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
Using the periodic table and your knowledge of patterns and trends on the table, which of the following elements is the most reactive?
A. Titanium (Ti, #22)
B. Silicon (Si, #14)
C. Oxygen (0, #8)
D. Argon (Ar, #18)
Answers
Based on the periodic trends in the periodic table, the most reactive element is oxygen; option C
What are periodic trends in the periodic table?Periodic trends in the periodic table refers to the periodic variation in the properties of the elements which is observed in the periodic table as one moves across the periodic table from left to right across a period or down a group in the periodic table.
This regular variation is also known as periodicity in the properties of elements.
Some of the periodic trends observed in the periodic table include:
the reactivity of metals increase down a group and from right to left across a periodthe reactivity of non-metals increase in a group from down to top and from left to right across a period across a period.Considering the most reactive element based on the periodic trends:
Titanium, Ti is a transition metal whose reactivity is intermediate as it is found in between group 2 and 3 of the periodic table
Silicon is metalloid which is not very reactive
Oxygen, O is a very reactive non-metal found in group 6A
Argon, is a noble gas which is almost inert.
Therefore, the most reactive element is oxygen.
Learn more about periodic trends at: brainly.com/question/28161428
#SPJ1
A burning match will burn more vigorously in pure oxygen than in air because _________ . Select one: a. oxygen is a catalyst for combustion b. nitrogen is a reactant in combustion and its low concentration in pure oxygen catalyzes the combustion c. oxygen is a product of combustion d. nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature e. oxygen is a reactant in combustion and pure oxygen increases the reactant concentration
Answers
Answer:
e. oxygen is a reactant in combustion and pure oxygen increases the reactant concentration
Explanation:
The reaction of a burning match is combustion. In this combustion, the organic components of the match (such as cellulose, C₆H₁₀O₅) react with oxygen, producing water and carbon dioxide:
C₆H₁₀O₅(s) + 6O₂(g) → 5H₂O(g) + 6CO₂(g)Seeing as oxygen is a reactant and not a catalyst nor product, and that nitrogen plays no part in the reaction, the only correct answer is option e.
100 POINTS!!!
What is the average rate of the reaction over the entire course of the reaction?
1.6 × 10−3 (?)
1.9 × 10−3 (?)
2.0 × 10−3 (X)
2.2 × 10−3 (X)

Answers
Answer:
b. 1.9 × 10-3
Explanation:
Answer:1.9x10-3
Explanation:
average
You walk into the lab, and you find a beaker sitting on the bench labeled HNO3. However, the concentration is not given. Your instructor tells you to do a titration to determine the concentration of the acid. You find that is takes 27.60 mL of 1.00 M NaOH to neutralize 10.00 of the HNO3. What is the concentration oft the HNO3?
HNO3 + NaOH
H2O + NaNO3
Answers
The concentration of the HNO₃ solution needed to neutralize the 27.60 mL of 1.00 M NaOH is 2.76 M
How do i determine the concentration of the HNO₃ solution?The balanced equtaion is given below:
HNO₃ + NaOH —> H₂O + NaNO₃
Mole ratio of the HNO₃ (nA) = 1Mole ratio of the NaOH (nB) = 1Now, we shall obtain the concentration of the HNO₃ solution needed for the neutralization reaction. This is shown below:
Volume of HNO₃ (Va) = 10 mLVolume of NaOH (Vb) = 27.60 mLConcentration of NaOH (Cb) = 1.00 M Concentration of HNO₃ (Ca) =?CaVa / CbVb = nA / nB
(Ca × 10) / (1 × 27.6) = 1
(Ca × 10) / 27.6 = 1
Cross multiply
Ca × 10 = 27.6
Divide both side by 10
Ca = 27.6 / 10
Ca = 2.76 M
Thus, the concentration of the HNO₃ solution needed is 2.76 M
Learn more about titration:
https://brainly.com/question/27817549
#SPJ1
When 50.5 g iron(III) oxide reacts with carbon monoxide, 32.2 g iron is produced. What is the percent yield of the reaction?
Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g)
Answers
Taking into account definition of percent yield, the percent yield for the reaction is 91.17%.
Reaction stoichiometryIn first place, the balanced reaction is:
Fe₂O₃ + 3 CO → 2 Fe + 3 CO₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Fe₂O₃: 1 mole CO: 3 moles Fe: 2 moles CO₂: 3 molesThe molar mass of the compounds is:
Fe₂O₃: 159.7 g/moleCO: 28 g/moleFe: 55.85 g/moleCO₂: 44 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Fe₂O₃: 1 mole ×159.7 g/mole= 159.7 grams CO: 3 moles ×28 g/mole= 84 gramsFe: 2 moles ×55.85 g/mole= 111.7 gramsCO₂: 3 moles ×44 g/mole= 132 gramsMass of Fe formedThe following rule of three can be applied: if by reaction stoichiometry 159.7 grams of Fe₂O₃ form 111.7 grams of Fe, 50.5 grams of Fe₂O₃ form how much mass of Fe?
\(mass of Fe=\frac{50.5 grams of Fe_{2} O_{3} x111.7 grams of Fe}{159.7 grams of Fe_{2} O_{3}}\)
mass of Fe= 35.32 grams
Then, 35.32 grams of Fe can be produced from 50.5 g iron(III) oxide.
Percent yieldThe percent yield is the ratio of the actual return to the theoretical return expressed as a percentage.
The percent yield is calculated as the experimental yield divided by the theoretical yield multiplied by 100%:
\(percent yield=\frac{actual yield}{theorical yield}x100\)
where the theoretical yield is the amount of product acquired through the complete conversion of all reagents in the final product, that is, it is the maximum amount of product that could be formed from the given amounts of reagents.
Percent yield for the reaction in this caseIn this case, you know:
actual yield= 32.2 gramstheorical yield= 35.32 gramsReplacing in the definition of percent yields:
\(percent yield=\frac{32.2grams}{35.32grams}x100\)
Solving:
percent yield= 91.17%
Finally, the percent yield for the reaction is 91.17%.
Learn more about
the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
percent yield:
brainly.com/question/14408642
#SPJ1
A hot metal plate at 150°C has been placed in air at room temperature. Which event would most likely take place over the next few minutes?
-Molecules in both the metal and the surrounding air will start moving at lower speeds. -Molecules in both the metal and the surrounding air will start moving at higher speeds. -The air molecules that are surrounding the metal will slow down, and the molecules in the metal will speed up.
-The air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down.
Answers
Answer:
until the next harvest, and seed must be held for the next season's ... successful grain storage is the moisture content of the crop. ... or both. If ambient temperatures are low, then air alone may cool the ... allow some of the drying to take place naturally while the crop ... employed to cool grain that has been placed in storage.
Perform the calculation, rounding your answer to the proper number of significant figures.
0.867 +3.72 + 18.0045 =
Answers
Answer:
Hey!
0.867 + 3.72 + 18.0045 = 22.5915
Explanation:
22.5915 ROUNDED TO 2 significant figures...
2 s.f = 23
(3 s.f = 22.6)
SO ROUNDING TO 2 s.f is easier and more accurate...
ANSWER = 23 (2 s.f)
HOPE THIS HELPS!!
The calculation, rounding your answer to the proper number of significant figures would be 22.60.
0.867 +3.72 + 18.0045 =22.60
What are significant figures?
In positional notation, significant figures refer to the digits in a number that is trustworthy and required to denote the amount of something, also known as the significant digits, precision, or resolution.
As given in the problem we have to perform the calculation, by rounding the answer to the proper number of significant figures.
0.867 +3.72 + 18.0045 =22.5915
=22.59
=22.60
Thus, the calculation, rounding your answer to the proper number of significant figures would be 22.60.
To learn more about significant figures here, refer to the link;
brainly.com/question/14359464
#SPJ2
22. Which of the following unit symbols apply a x1000 multiplication factor?
A. km, kg, kl
B. m, g. 1
C. cm, cg, cl
D. mm, mg, ml
Answers
A beaker contains a total of 500 ml of solution which is 0.00050 M Ag^+, 0.00050 M Pb^2+, and 0.00050 M in Mn^2+ ions. If 10.00 ml of 1.0*10^-6 M Na2CO3 is added to the beaker, what will precipitate?
Ksp Ag2CO3 = 8.1*10^-12
Ksp PbCO3 = 7.4*10^-14
Ksp MnCO3 = 8.8*10^-11
Answers
Only Ag2CO3 will precipitate from the solution.
Precipitation reactionWhen Na2CO3 is added to the solution, it will react with the Ag^+ and Pb^2+ ions to form precipitates of Ag2CO3 and PbCO3. The Mn^2+ ion concentration is not high enough to form a precipitate with Na2CO3.
First, let's calculate the initial concentration of Ag^+ and Pb^2+ ions in the solution:
Ag^+: 0.00050 M
Pb^2+: 0.00050 M
Next, we need to calculate the concentration of Na2CO3 after it is added to the solution. Since we added 10.00 ml of 1.0*10^-6 M Na2CO3 to a total volume of 500 ml, the final concentration of Na2CO3 is:
[Na2CO3] = (10.00 ml / 500 ml) * 1.010^-6 M
[Na2CO3] = 2.010^-8 M
Now we can use the Ksp values to determine which precipitates will form.
For Ag2CO3:
Ksp = [Ag^+]^2[CO3^2-]
8.110^-12 = (2x)^2 (2x)
8.110^-12 = 4x^3
x = 2.0*10^-4 M
Since the concentration of CO3^2- is higher than the solubility product, Ag2CO3 will precipitate.
For PbCO3:
Ksp = [Pb^2+][CO3^2-]
7.410^-14 = (0.00050 M)(2x)
x = 9.210^-11 M
Since the concentration of CO3^2- is lower than the solubility product, PbCO3 will not precipitate.
Therefore, the only precipitate that will form is Ag2CO3.
More on precipitation reactions can be found here: https://brainly.com/question/24158764
#SPJ1