in a 74.0-g 74.0 -g aqueous solution of methanol, ch4o, ch 4 o , the mole fraction of methanol is 0.170. 0.170. what is the mass of each component?
Answers
Given,Aqueous solution of Methanol Mass of the aqueous solution, wA = 74.0 gMole fraction of Methanol, XA = 0.170We are to find,Mass of Methanol, wBMass of Water.
The mole fraction of Methanol is defined as the number of moles of methanol divided by the total number of moles of all components (methanol + water).Hence,Number of moles of Methanol, nA = XA * nBTaking nB = 1,Number of moles of Methanol .
Applying the mole concept,Mass of Methanol, wB = nA Molar Mass of Methanol= 0.170 mol * 32.04 g mol⁻¹ = 5.45 gMass of Water, wC = wA - wB= 74.0 g - 5.45 g = 68.55 g Therefore,Mass of Methanol = 5.45 gMass of Water = 68.55 g.
To know more about Methanol Mass visit :
https://brainly.com/question/30902861
#SPJ11
Related Questions
which of the following molecules will experience the greatest dispersion forces? group of answer choices ch3ch2ch2ch3 ch3ch2ch2ch2ch2ch3 ch3ch2oh ch3ch2och2ch3
Answers
Among the molecules provided, the one experiencing the greatest dispersion forces is CH₃CH₂CH₂CH₂CH₂CH₃.
Dispersion forces, also known as London dispersion forces or van der Waals forces, are temporary attractive forces that result from fluctuations in electron distribution within molecules.
CH₃CH₂CH₂CH₂CH₂CH₃, also known as hexane, has the longest carbon chain and therefore the largest surface area. A larger surface area enables more opportunities for interaction between molecules, resulting in stronger dispersion forces. Additionally, hexane is a nonpolar molecule, so its primary intermolecular forces are dispersion forces.
The other two molecules, CH₃CH₂CH₂CH₃ (butane) and CH₃CH₂OCH₂CH₃ (diethyl ether), have shorter carbon chains, leading to smaller surface areas and weaker dispersion forces. Additionally, CH₃CH₂OH (ethanol) is not included in the list of molecules provided, but it is worth mentioning that it has hydrogen bonding due to the presence of an OH group, making its intermolecular forces different from the others.
In summary, CH₃CH₂CH₂CH₂CH₂CH₃ (hexane) experiences the greatest dispersion forces due to its longer carbon chain, larger surface area, and nonpolar nature.
Learn more about Dispersion forces here: https://brainly.com/question/14263432
#SPJ11
Complete the following statement. An energized atom of a particular element emits light by: emitting a number of photons so that the sum of their energies corresponds to the amount of energy lost by the atom. emitting a photon whose velocity depends on the amount of energy los. emitting one photon whose wavelength is related to the amount of energy lost by the atom. losing an electron whose velocity depends on the amount of energy lost by the atom. emitting brighter light as the amount of energy lost increases.
Answers
An energized atom emits light by releasing the excess energy as a photon whose wavelength is related to the amount of energy lost by the atom.
An energized atom of a particular element emits light by emitting a photon whose wavelength is related to the amount of energy lost by the atom. When an atom is excited by absorbing energy, such as heat or electrical energy, it moves to a higher energy level or excited state. The atom then releases the excess energy by emitting a photon of light as it returns to a lower energy level or ground state.The energy of a photon is directly proportional to its frequency and inversely proportional to its wavelength. Therefore, when an atom loses energy by emitting a photon, the wavelength of the emitted light is related to the amount of energy lost by the atom. The shorter the wavelength, the higher the energy of the emitted photon.Each element has a unique set of energy levels or orbitals, and when an atom of a particular element is excited, it emits light of specific wavelengths, which can be used to identify the element. This is the basis of spectroscopy, a technique that is widely used in chemistry, physics, and astronomy.In summary, an energized atom emits light by releasing the excess energy as a photon whose wavelength is related to the amount of energy lost by the atom.For more such question on wavelength
https://brainly.com/question/28995449
#SPJ11
A student measured the gram weight of a
metal object to be 93.5g. According to the
supplier the object weighs 93.9g. What is the
error in the student's measurement?
Answers
Answer: 0.428%
Explanation: % Error = [(measured - Actual)/Actual]*100%
(93.9g - 93.5 g)/93.5g = 0.428%
Answer:
+0.4
Explanation:
For all those Acellus kids XD
The melting point of this substance is __________________.
Put a number in the answer.

Answers
The melting point of the substance is 12.5 degrees Celsius
What is melting pointMelting point is the temperature at which a solid substance changes state from solid to liquid at atmospheric pressure.
At the melting point, the solid and liquid phases of the substance exist in equilibrium, and any further increase in temperature will cause the substance to completely melt into a liquid.
The melting point is a characteristic physical property of a substance and can be used to identify and purify a substance. The melting point of a substance is usually reported in degrees Celsius (°C) or Kelvin (K).
Learn more about melting point at
https://brainly.com/question/40140
#SPJ1
what is the similarity between man and woman
Answers
Help me pls ASAP if you know it thank you!

Answers
Answer:ASEXUAL: Single parent, Genetically identical, Replication,Budding, splitting / splicing and plant cutting propogation SEXUAL: Two parents, genetically unique, Humans and Cows
Explanationasexual is one parent or stays the same, sexual is where two combine
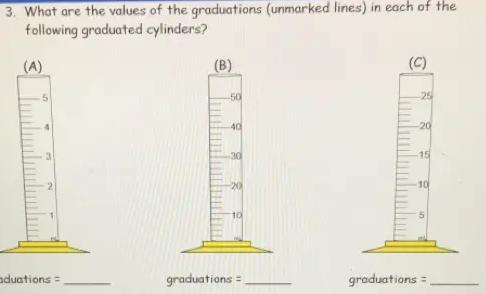
3. What are the values of the graduations (unmarked lines) in each of the
following graduated cylinders?
Q
(A)
50
3
graduations =
(B)
50
-40
-30
-20
10
graduations =
(C)
25
-20
-15
10
5
graduations =
Answers
The values of the Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
Graduated Cylinder - A popular piece of scientific equipment used to measure the volume of a liquid is a graduated cylinder, sometimes referred to as a measuring cylinder or mixing cylinder. Its form is slender and cylindrical. Each marked line on the graduated cylinder indicates the volume of liquid that has been measured.
The clear graduated cylinder is widely used for volume measurements and is regarded to be more accurate than a beaker since it contains permanently marked incremental graduations.
To calculate the value of graduations-
1. Subtract the two values to get the value between the labeled graduations.
2. Determine how many spaces there are between the two graduations. Keep in mind that volume equals space.
3. Divide the number of gaps by the value between the graduations.
For cylinder A.
2 ml- 1ml = 1ml
Number of spaces = 5
∴1/ 5 = 0.2
Graduation = 0.2ml
For cylinder B -
20-10 = 10
number of spaces = 5
∴10/ 5 =2
Graduation = 2ml
For cylinder C-
10-5 = 5
Number of spaces= 5
∴5/5 = 1
Graduation = 1ml
Hence , Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
To learn more about graduated cylinders refer-https://brainly.com/question/26216613
#SPJ9
Your question is incomplete. Please find the missing image below.
Calculate 8D of water vapor in isotopic equilib- rium with fresh water whose 8D value is -65%0, assuming that a (liquid-vapor) = 1.090.
Answers
The value of 8D of water vapor in isotopic equilibrium with fresh water whose 8D value is -65%0 is -67.79125‰.
The expression for calculating 8D of water vapor in isotopic equilibrium with fresh water can be given by: 8D = α 8D (vapor) + (1 - α) 8D (liquid). Where,α is a fractionation factor and 8D (vapor) and 8D (liquid) are the deuterium enrichments in water vapor and liquid, respectively.
The value of α is given by:a (liquid-vapor) = 1.090So,α = (a (liquid-vapor) - 1) / (a (liquid-vapor) + 1)α = (1.090 - 1) / (1.090 + 1)α = 0.045So,8D = α 8D (vapor) + (1 - α) 8D (liquid)Given,8D (liquid) = -65‰ (‰ denotes permil, which is equal to parts per thousand)
Substitute the given values in the expression and simplify:8D = 0.045 × 8D (vapor) + (1 - 0.045) × (-65)8D = 0.045 × 8D (vapor) - 61.9258D + 2.79125 = 8D (vapor)
Therefore,8D (vapor) = 8D - 2.79125= -65 - 2.79125= -67.79125‰ (answer)Therefore, the value of 8D of water vapor in isotopic equilibrium with fresh water whose 8D value is -65%0 is -67.79125‰.
To learn more about vapor visit;
https://brainly.com/question/32499566
#SPJ11
How long does it take for the water to start boiling? At what temperature does the water boil?
Answers
Answer:
At sea level, or at zero feet in altitude, the boiling point of water is at 212 °F (100 °C). Once the water has reached this boiling point, the US Center for Disease Control recommends keeping it at a rolling boil for about a minute to make sure it is purified.
Answer:
Water boils at 212 degrees fahrenheit or 100 degree celcius
Explanation:
its true
Elements are made up of one type of atom. Compounds are particles that have more than one atom joined together. They can be ........ or ......
Answers
Answer:
metal or non metal
Explanation:
this is the answer
Which object has the most potential energy? A ball resting on the ground. A ball being thrown at 100 miles per hour. A ball on top of a refrigerator. A ball resting on the edge of a cliff.
Answers
Answer:
The correct option is a ball resting on the edge of a cliff.
Explanation:
A ball resting on the edge of a cliff is the correct option because it has the most potential energy and this is because potential energy is the energy stored by an object or energy posses by an object due it is position. It is the energy at rest.
A ball resting on the edge of a cliff indicate that the ball is a t rest, and it's possess energy base on its position relative to it's zero position.
Answer:A ball resting on the edge of a cliff.
Explanation:
How will you test for the gas which is liberated when hcl reacts with an active metal ?
Answers
pyroxene has the chemical formula, (naca)(mg,fe,al)(al,si)2o6. is pyroxene a felsic or mafic silicate mineral?
Answers
pyroxene has the chemical formula, (naca)(mg,fe,al)(al,si)2o6. pyroxene is a Mafic silicate mineral.
silicate mineral or extrusive igneous rich in iron and magnesium is widely recognized as a mafic mineral or rock. The large percentage of mafic minerals are dark, and some of the most common rock-forming mafic minerals are olivine, pyroxene, amphibole, and biotite. Basalt, diabase, and gabbro are examples of common mafic rocks. When trying to discuss stones or lava, mafic refers to the quantity of silica inside the lava or rock, whereas felsic refers to the amount of silica in the lava or stone. 6. Mafic rocks are darker in colour than felsic rocks. A chemical formula is a way to convey information about the chemical percentages of atoms that make up a specific chemical compound and molecule by using chlorine atom symbols, numbers, and occasionally other symbols like parentheses, dashes, brackets, commas, and plus and minus signage.
Learn more about silicate mineral here:
https://brainly.com/question/6868721
#SPJ4
The oxidation of inorganic molecules such as hydrogen sulfide into carbohydrates is called:
a. photosynthesis.
b. chemosynthesis.
c. photoautotrophy.
d. evolution.
Answers
it is called chemosynthesis
The oxidation of inorganic molecules such as hydrogen sulfide into carbohydrates is called chemosynthesis.
option B is the correct answer.
What is oxidation?Oxidation is a process in which a chemical substance changes because of the addition of oxygen. Carbon dioxide is a necessary result of the oxidation of carbon compounds.
Chemosynthesis is the biological conversion of one or more carbon-containing molecules and nutrients into organic matter using the oxidation of inorganic compounds or ferrous ions as a source of energy, rather than sunlight, as in photosynthesis.
So the oxidation of inorganic molecules such as hydrogen sulfide into carbohydrates is called chemosynthesis.
Learn more about oxidation here: https://brainly.com/question/13182308
#SPJ4
consider the methane molecule. what is the central atom? enter its chemical symbol. how many lone pairs are around the central atom? what is the ideal angle between the carbon-hydrogen bonds? compared to the ideal angle, you would expect the actual angle between the carbon-hydrogen bonds to be ... (choose one)
Answers
The methane molecule is CH₄, the central atom in the methane molecule is Carbon , C. there is zero lone pair on the carbon atom. the angle between the carbon - hydrogen is 109.5 °C.
The methane molecule with molecular formula is given as :
CH₄ , the no. of the lone pairs in the methane molecule on the central atom carbon is zero lone pair. the bond pair in the methane molecule is the four. the molecular geometry of the methane molecule is tetrahedral. the hybridization of methane is sp³. the ideal bond angle between the carbon-hydrogen bonds is 109.5 °C.
The methane, CH₄ molecule is formed by the covalent bond and this is a covalent compound.
To learn more about methane here
https://brainly.com/question/2127750
#SPJ4
Air trapped in a cylinder fitted with a piston occupies 145.7 mL at 1.08 atm pressure.
What is the new volume when the piston is depressed, increasing the pressure by 25%
Answers
A mixture of H2 and water vapor is present in a closed vessel at 20°C. The total pressure of the system is 755. 0 mmHg. Partial pressure of water vapor at 20°C equals 17. 5 mmHg. What is the partial pressure of H2? mmHg.
Answers
The partial pressure of hydrogen in the mixture has been 737.5 mm Hg.
The partial pressure has been defined as the pressure exerted by the individual gas in the mixture.
According to the Dalton's law of partial pressure, the total pressure of the gas, has been the sum of the partial pressure of constituents gas.
Computation for the partial pressure of HydrogenThe given mixture has been the combination of hydrogen and water vapor. According to the Dalton's law, the total pressure, P of the system has been:
\(P=P_H_2\;+\;P_{H_2O}\)
The total pressure of the gas has been, \(P=755\;\rm mm\;Hg\)
The partial pressure of water vapor, \(P_{H_2O}=17.5\;\rm mm\;Hg\)
Substituting the values for the partial pressure of hydrogen,\(P_{H_2}\)
\(755=P_{H_2}\;+\;17.5\;\text{mm Hg}\\P_{H_2}=755-17.5\;\text{mm Hg}\\P_{H_2}=737.5\;\rm mm\;Hg\)
The partial pressure of hydrogen in the mixture has been 737.5 mm Hg.
Learn more about partial pressure, here:
https://brainly.com/question/14623719
the momentum of a rock is increased by 0.724kgm/s when a force of 0.891n is applied to it. how long does it take
Answers
Therefore, it takes approximately 0.813 seconds for the momentum of the rock to be increased by 0.724 kg·m/s when a force of 0.891 N is applied to it.
To calculate the time it takes for the momentum of a rock to be increased by 0.724 kg·m/s when a force of 0.891 N is applied to it, we can use the following equation:
Δp = F * Δt
where Δp is the change in momentum, F is the force applied, and Δt is the time it takes for the force to be applied.
In this case, the force applied is 0.891 N and the momentum is increased by 0.724 kg·m/s. Therefore, we can write:
Δp = F * Δt
Δp = 0.891 N * 0.724 kg·m/s
Δp = 6.67 kg·m/s
Δt = Δp / F
Δt = 6.67 kg·m/s / 0.891 N
Δt = 0.813s
Learn more about momentum visit: brainly.com/question/18798405
#SPJ11
.Which of the following is not a part of Dalton's atomic theory?
A
All matter is composed of indivisible atoms.
B
The properties of atoms of the same element can be different.
C
Compounds form when atoms combine in whole number ratios.
D
An atom can neither be created nor destroyed.
Answers
The answer is B - The properties of atoms of the same element can be different. This is not a part of Dalton's atomic theory. According to Dalton's atomic theory, all matter is composed of indivisible atoms, compounds form when atoms combine in whole number ratios, and an atom can neither be created nor destroyed.
Your answer: B - The properties of atoms of the same element can be different.
This statement is not a part of Dalton's atomic theory, as according to his theory, atoms of the same element have the same properties.
to know more about properties intake pls visit:
https://brainly.com/question/14951376
#SPJ11
14. Aluminum reacts with a certain unknown element (element X) to form the following formula. Which group must element X belong to? *

Answers
Answer:
c
Explanation:
Draw the atomic structure of sodium with its electronic configuration.
Answers
here you go. kindly check attatchement

which reaction will occur? 2NaBr+I2 —> 2NaI + Br2
Answers
Answer:
This is a single replacement reaction because I replaces Br.
Answer: This reaction can'toccur
Explanation
Iodine can't displace Bromine from its compound been less reactive than Br
In the previous step, you determined
0.25 mol HCI reacts. The molar mass
of Mg is 24.31 g/mol.
What mass of Mg is required?
PLEASE HELP ASAP

Answers
Approximately 3.04 grams of magnesium would be required to react with 0.25 moles of hydrochloric acid.
To determine the mass of Mg required, we need to use the balanced chemical equation for the reaction between hydrochloric acid (HCl) and magnesium (Mg):
2HCl + Mg → MgCl2 + H2
From the balanced equation, we can see that 2 moles of HCl react with 1 mole of Mg. Therefore, if 0.25 mol of HCl reacts, we would need half of that amount, which is 0.125 mol of Mg.
To calculate the mass of Mg required, we need to multiply the number of moles of Mg by its molar mass. The molar mass of Mg is given as 24.31 g/mol. Therefore, the mass of Mg required can be calculated as follows:
Mass of Mg = Number of moles of Mg × Molar mass of Mg
Mass of Mg = 0.125 mol × 24.31 g/mol
Mass of Mg = 3.04 g
For such more questions on moles
https://brainly.com/question/19964502
#SPJ8
Which of these properties is most helpful when identifying a substance in a
given sample of matter?
A. State
B. Mass
C. Melting point
D. Volume
Answers
Answer:
Melting point
Explanation:
Melting point is a intensive property and Intensive properties can be used to help identify a sample because these characteristics do not depend on the amount of sample, nor do they change according to conditions.
IF IT WAS HELPFUL HIT THE CROWN
Answer:
Explanation:
Yes melting point!
Describe the sludge generation process and propose safe methods
of disposing it.
Answers
The sludge generation process refers to the production of sewage treatment residue during wastewater treatment. Sludge contains solid and semi-solid materials that must be handled and disposed of properly to protect human health and the environment.
The following are some methods for sewage disposal:
Wastewater Treatment: Initial treatment involves the physical removal of large solids, whereas secondary treatment uses biological processes to break down organic matter and remove dissolved pollutants.
Sludge Treatment: The separated sludge is under further treatment, which may include stabilization, dewatering, and, in some cases, additional processes to reduce contaminants.
Land Application: Treated sludge can be applied to agricultural land as a fertilizer or soil conditioner if it meets regulatory guidelines and has been properly treated.
Landfills: If sludge cannot be reused or recycled, it can be disposed of in a designated landfill that meets regulatory requirements, ensuring proper containment and preventing soil and water contamination.
For more information regarding sludge and sewage disposal:
https://brainly.in/question/8504457?utm_source=android&utm_medium=share&utm_campaign=question
A 0.755-g sample of hydrated copper(II) sulfate CuSO4 · xH2O was heated carefully until it had changed completely to anhydrous copper(II) sulfate (CuSO4) with a mass of 0.483 g. Determine the value of x. [This number is called the number of waters of hydration of copper(II) sulfate. It specifies the number of water molecules per formula unit of CuSO4 in the hydrated crystal.]
Answers
There are five molecules of the water of crystallization.
Mass of hydrated copper(II) sulfate CuSO4 · xH2O = 0.755 g
Mass of anhydrous copper(II) sulfate CuSO4 = 0.483 g
Molar mass of hydrated copper(II) sulfate CuSO4 · xH2O = (160 + 18x) g/mol
Molar mass of anhydrous copper(II) sulfate CuSO4 =160 g/mol
Number of moles of hydrated copper(II) sulfate CuSO4 · xH2O = Number of moles of anhydrous copper(II) sulfate CuSO4
Therefore;
0.755 g/(160 + 18x) g/mol = 0.483 g/160 g/mol
0.755 * 160 = 0.483 (160 + 18x)
120.8 = 77.28 * 8.694x
120.8 - 77.28 = 8.694x
x = (120.8 - 77.28)/8.694
x = 5
The formula of the hydrated salt is CuSO4 · 5H2O.
Learn more; https://brainly.com/question/14252791
What is the v if = 8 m and f = 20 Hz?
Answers
Answer:
Explanation:
it is 980
how did Bohr change Rutherford's model
Answers
Explanation:
To solve that deadlock problem, in 1913 Bo kept Rizopho's model of planetary atom and combined with Plang quantum theory, he produced a hydrogen atom model with the following three determinations: - In atoms, The clectron cannot orbit any other, but only rotates with a certain number of orbits. Each "allowed" orbit is equivalent to a certain energy. - When rotating in "allowed" orbits, electrons do not lose energy, meaning they do not emit radiation, but emit radiation only when the electron jumps from an orbit with the high energy level in the orbit corresponding to the the low energy and the household energy of radiation are equal to the difference of the two energy levels. - - When rotating in those "allowed" orbits, the electron has a momentum moment that is an integer times of h / (2π).
scientists collect evidence by making. of the world around them
Answers
Answer:
By making sense of the World around them
Explanation:
maybe?
What is the 3D shape of H3o+
Answers
3D view of H3O+
The stronger line means that the Hydrogen is closer to us in a 3D view, meanwhile the line with traces, or you cal also see as a weaker line, it means that the Hydrogen is farther from us in a 3D view
Lewis Structure of H3O+

