Answers
Answer:
2 days.
Explanation:
Related Questions
Please show work, thanks!

Answers
In chemistry, “partial pressure” refers to the pressure that each gas in a gas mixture exerts against its surroundings, such as a sample flask, a diver’s air tank, or the boundary of an atmosphere. You can calculate the pressure of each gas in a mixture if you know how much of it there is, what volume it takes up, and its temperature. You can then add these partial pressures together to find the total pressure of the gas mixture, or, you can find the total pressure first and then find the partial pressures.
6.00 moles of sodium perchlorate contains the same number of atoms as __________. 6.00 moles of sodium perchlorate contains the same number of atoms as __________. 6.00 moles of sodium permanganate 6.00 moles of strontium oxide 6.00 moles of sodium cyanide 6.00 moles of lithium phosphate 6.00 moles of sodium nitrite
Answers
Answer:
6.00 moles of sodium permanganate
Explanation:
Let us attempt to count the number of atoms present in sodium perchlorate. The formula of the compound is NaClO4
Sodium atoms - 1
Chlorine atoms - 1
Oxygen atoms -4
Total number of atoms = 6
Among the options, only KMnO4 also has six atoms. One atom of potassium, one atom of manganese and four atoms of oxygen.
6.00 moles of sodium perchlorate contains the same number of atoms as: A. 6.00 moles of sodium permanganate.
How to calculate the number of atoms.First of all, we would determine the chemical formula and the number of atoms contained in each chemical compound as follows:
For sodium perchlorate:
Sodium perchlorate = \(NaClO_4\)
\(NaClO_4\) = \(1+1+2\) = 6 atoms.
For sodium permanganate:
Sodium permanganate = \(KMnO_4\)
\(KMnO_4\) = \(1+1+2\) = 6 atoms.
For strontium oxide:
Strontium oxide = \(SrO\)
\(SrO\) = \(1+1\) = 2 atoms.
In conclusion, we can deduce that 6.00 moles of sodium perchlorate contains the same number of atoms as 6.00 moles of sodium permanganate.
Read more on number of moles here: https://brainly.com/question/3173452
50 Points (Brainliest too)! Please help!
1. In detail, explain what transmutation is.
2. In one to two sentences, explain what an alpha particle is.
3. Explain the difference between a chemical equation and a nuclear equation.
4. What is the relationship between the atomic number and the mass number of a 5. nucleus?
Please help me understand these!! I'm really confused!
Answers
Answer:
Transmutation is the conversion of an atom of one element into an atom of a different element through nuclear changes.
An alpha particle is a particle composed of 2 protons and 3 neutrons. It results from transmutation because of the change in protons in a large nucleus.
A chemical equation is balanced accord to the number of atoms of each element before and after the change. This is also shows the Law of Conservation of Matter.
A nuclear equation is balanced according to mass number and charge (atomic number). These equations can’t be balanced like chemical equations because the identities of the atoms can change. On the left of equation, the top number is the atomic mass and the bottom number is the atomic number. Take note that if you add the atomic mass (top number), you’ll get the the atomic mass of of the atom pre-transmutation. Same applies with atomic number. This shows a balanced nuclear equation.
The atomic number is the number of protons in a nucleus. The mass number is the sum of the protons and neutrons in a nucleus. Electrons have very small masses, so they are not accounted in atomic mass.
Part A
How much heat is required to convert 4.88 g of ice at-14.0°C to water at 23.0°C? (The heat capacity of ice is 2.09 J/(g- "C). AHp (H₂O) = 40.7 kJ/mol, and AH (H₂O) = 6.02 kJ/mol.)
Express your answer with the appropriate units.
?
Answers
Taking into account the definition of calorimetry, sensible heat and latent heat, the amount of heat required is 2.243974 kJ or 2,243.974 J.
Definition of calorimetry, sensible heat and latent heatCalorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
Sensible heat is defined as the amount of heat that a body absorbs or releases without any changes in its physical state (phase change).
Latent heat is defined as the energy required by a quantity of substance to change state.
When this change consists of changing from a solid to a liquid phase, it is called heat of fusion and when the change occurs from a liquid to a gaseous state, it is called heat of vaporization.
-14 °C to 0°CIn firts place, you know that the melting point is 0°C. So, first of all you must increase the temperature from -14 ° C (in solid state) to 0 ° C, in order to supply heat without changing state (sensible heat).
The amount of heat a body receives or transmits is determined by:
Q = c× m× ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case, you know:
c(solid)= heat capacity of ice= 2.09 \(\frac{J}{gC}\)m= 4.88 gΔT= Tfinal - Tinitial= 0 °C - (-14) °C= 14 °CReplacing:
Q1= 2.09 \(\frac{J}{gC}\) × 4.88 g× 14 °C
Solving:
Q1=142.7888 J= 0.1427888 kJ
Change of stateThe heat Q that is necessary to provide for a mass m of a certain substance to change phase is equal to
Q = m×L
where L is called the latent heat of the substance and depends on the type of phase change.
In this case, you know:
n= 4.88 g×\(\frac{1 mole}{18 g}\) = 0.2711 moles, where 18 \(\frac{g}{mole}\) is the molar mass of H₂O, that is, the amount of mass that a substance contains in one mole.ΔHfus= 6.02 \(\frac{kJ}{mol}\)Replacing:
Q2= 0.2711 moles×6.02 \(\frac{kJ}{mol}\)
Solving:
Q2= 1.632022 kJ
0 °C to 23 °CSimilar to sensible heat previously calculated, you know:
c(liquid)= specific heat of water= 4.18\(\frac{J}{gC}\)m= 4.88 gΔT= Tfinal - Tinitial= 23 °C - 0 °C= 23 °CReplacing:
Q3= 4.18\(\frac{J}{gC}\) × 4.88 g× 23 °C
Solving:
Q3= 469.1632 J= 0.4691632 kJ
Total heat requiredThe total heat required is calculated as:
Total heat required= 0.1427888 kJ + 1.632022 kJ + 0.4691632 kJ
Total heat required= 2.243974 kJ= 2,243.974 J
In summary, the amount of heat required is 2.243974 kJ or 2,243.974 J.
Learn more about calorimetry:
brainly.com/question/14057615
brainly.com/question/24988785
brainly.com/question/21315372
brainly.com/question/13959344
brainly.com/question/14309811
brainly.com/question/23578297
#SPJ1
3350 J of heat is required to raise the temperature of a sample of AlF3 from 25°C to 80°C. What is the mass of the sample?
(Format: XX.XX)
Answers
The mass of the sample of AlF₃ is 6.10 g.
To calculate the mass of the sample of AlF₃, we can use the formula: Q = m × c × ΔT
where Q is the amount of heat required, m is the mass of the sample, c is the specific heat capacity of AlF₃, and ΔT is the change in temperature. We can rearrange this formula to solve for the mass of the sample: m = Q / (c × ΔT)
We are given Q = 3350 J, ΔT = 80°C - 25°C = 55°C, and the specific heat capacity of AlF₃ is 1.024 J/g·°C. Substituting these values into the formula, we get: m = 3350 J / (1.024 J/g·°C × 55°C) = 6.10 g
Therefore, the mass of the sample of AlF₃ is 6.10 g.
To learn more about specific heat capacity here:
https://brainly.com/question/29766819
#SPJ1
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
What is the main point of paragraph 3?
A Red blood cells
B the skeletal system
C the structure of a bone
D bones, cartilage, and blood vessels

Answers
hi there, giving brainliest to best answer. no spammers

Answers
Answer:
D. Earth's northern hemisphere is tilted toward the Sun
Explanation:
You can see in the picture above that the northern hemisphere is past the perpendicular to orbit line to the right.
why is the sun earth and moon system important
Answers
The Sun-Earth-Moon system is important because it sustains life on Earth, regulates Earth's climate, and influences natural phenomena like tides.
The Sun-Earth-Moon system plays a vital role in supporting and sustaining life on Earth. The Sun is the primary source of energy for our planet, providing heat and light necessary for photosynthesis, the process by which plants convert sunlight into food and oxygen. Sunlight is also crucial for maintaining Earth's temperature and driving weather patterns.
The Moon, as Earth's only natural satellite, contributes to several essential functions. Its gravitational pull creates the tides, which influence coastal ecosystems and shape coastal landscapes.
The Moon's orbit also stabilizes Earth's axial tilt, providing a stable climate for life to thrive. Additionally, the Moon's phases have cultural and historical significance, influencing human activities such as agriculture, navigation, and calendar systems.
The Sun-Earth-Moon system's interactions are responsible for natural phenomena like eclipses, both solar and lunar, which have fascinated humans throughout history and continue to be important for scientific study and exploration.
Understanding these celestial events enhances our knowledge of astrophysics and helps us comprehend the vastness and complexity of the universe.
Furthermore, the study of the Sun-Earth-Moon system provides insights into celestial mechanics, orbital dynamics, and the broader field of planetary science. By examining the interplay between these celestial bodies, scientists can gain a deeper understanding of Earth's place in the universe and explore potential habitable conditions on other celestial bodies.
Overall, the Sun-Earth-Moon system is of immense importance as it sustains life, regulates climate, influences natural phenomena, and provides a platform for scientific exploration and discovery.
For more question on climate visit:
https://brainly.com/question/12801279
#SPJ8
Phương trình hóa học là gì
Answers
Explanation:
sự xuất bản hóa học nào? có một câu hỏi về khoa học mà bạn cần giúp đỡ? tôi luôn ở đây để giúp đỡ!
g match each toxic material with its most dangerous property. group of answer choices bromine [ choose ] mercury [ choose ] strong bases [ choose ] formaldehyde [ choose ] cyclic ethers [ choose ]
Answers
Toxic material with its most dangerous property:
bromine: corrosive
mercury: neurotoxicity
strong bases: severe skin and eye irritation/burns
formaldehyde: carcinogenicity
cyclic ethers: central nervous system depression.
Bromine is a toxic material that is highly corrosive, which means it can cause damage to the skin and eyes, as well as damage to internal organs if ingested.
Mercury is a toxic metal that is highly neurotoxic, meaning it can damage the nervous system and cause neurological symptoms such as tremors, memory loss, and difficulty walking.
Strong bases, such as lye, can cause severe skin and eye irritation and burns, and can also cause damage to internal organs if ingested.
Formaldehyde is a toxic chemical that is classified as a carcinogen, meaning it has the potential to cause cancer.
Cyclic ethers are a group of chemicals that are known to cause central nervous system depression, which can lead to symptoms such as drowsiness, confusion, and unconsciousness.
To know more about Bromine,
https://brainly.com/question/29301746
#SPJ4
If the number of moles of a gas doubles, its volume will also double
according to
*
Please hurry! What is the answer to this?

Answers
Explanation:
Boyle's Law
Find the mass of 3.9 x 1023 molecules of carbon dioxide gas at STP conditions. *
O 28.6 grams
O 67.7 grams
O 76.4 grams
O 19.1 grams
Answers
The molar mass (formula weight) of carbon dioxide (CO2) is 44.01 g/mol.
At STP (Standard Temperature and Pressure), which is defined as 0°C (273.15 K) and 1 atm of pressure, 1 mole of any gas occupies 22.4 L of volume.
Now, we have 3.9 x 10^23 molecules of CO2:
- To find the number of moles, we divide the number of molecules by Avogadro's number:
n = N/Na = 3.9 x 10^23/6.022 x 10^23 = 0.648 moles
- To find the mass, we multiply the number of moles by the molar mass:
mass = n x M = 0.648 mol x 44.01 g/mol = 28.52 grams
Therefore, the mass of 3.9 x 10^23 molecules of carbon dioxide gas at STP is approximately 28.6 grams (option A).
A solution contains 0.0150 M Pb2+(aq) and 0.0150 M Sr2+(aq) . If you add SO2−4(aq) , what will be the concentration of Pb2+(aq) when SrSO4(s) begins to precipitate?
Answers
Answer:
\(\large \boxed{1.10 \times 10^{-3}\text{ mol/L}}\)
Explanation:
1. Concentration of SO₄²⁻
SrSO₄(s) ⇌ Sr²⁺(aq) +SO₄²⁻(aq); Ksp = 3.44 × 10⁻⁷
0.0150 x
\(K_{sp} =\text{[Sr$^{2+}$][SO$_{4}^{2-}$]} = 0.0150x = 3.44 \times 10^{-7}\\x = \dfrac{3.44 \times 10^{-7}}{0.0150} = \mathbf{2.293 \times 10^{-5}} \textbf{ mol/L}\)
2. Concentration of Pb²⁺
PbSO₄(s) ⇌ Pb²⁺(aq) + SO₄²⁻(aq); Ksp = 2.53 × 10⁻⁸
x 2.293 × 10⁻⁵
\(K_{sp} =\text{[Pb$^{2+}$][SO$_{4}^{2-}$]} = x \times 2.293 \times 10^{-5} = 2.53 \times 10^{-8}\\\\x = \dfrac{2.53 \times 10^{-8}}{2.293 \times 10^{-5}} = \mathbf{1.10 \times 10^{-3}} \textbf{ mol/L}\\\\\text{The concentration of Pb$^{2+}$ is $\large \boxed{\mathbf{1.10 \times 10^{-3}}\textbf{ mol/L}}$}\)
KH D m d c mEJOg3.65 g = [?] mg=Enter
![KH D m d c mEJOg3.65 g = [?] mg=Enter](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/dN3cF8fLq4q7DluA8S9zZpj0KsVVmShz.jpeg)
Answers
In each 1 gram, we have 1000 miligrams, that is:
1 g = 1000 mg
So, we can use rule of three to find the answer:
x ---- 3.65 g
1000 mg ---- 1 g
Thus:
\(\begin{gathered} \frac{x}{1000mg}=\frac{3.65\cancel{g}}{1\cancel{g}} \\ x=1000mg\cdot3.65 \\ x=3650mg \end{gathered}\)So, we have:
3.65 g = 3650 mg
The water is colder than the sand because it requires more to change temperature. This is because the water has a higher than the sand.
Answers
The water is colder than the sand because it requires more to change temperature. This is because the Specific heat of water is more than sand.
Specific heat capacity is the amount of thermal energy required to raise the temperature of a substance. Water has a very high specific heat capacity. On the other hand, sand and asphalt have a low specific heat capacity. This means the temperature changes faster.
Sand should heat up and cool faster than water.
This is because water has a higher specific heat than sand, so it takes a lot of heat or energy to raise the temperature of water by 1 degree, whereas it takes relatively little energy to raise the temperature of sand by 1 degree.
And also,
When Brad was swimming in the ocean, he noticed that the sand was hotter than the water in the ocean.
Water is colder than sand because it takes more energy to change its temperature than sand.
This is because the specific heat of water is greater than that of sand. Due to this, sand cools and heats up faster than water.
Learn more about Water:
https://brainly.com/question/28465561
#SPJ4
If a light bulb has a voltage of 1.5 V and has 2.5A of current running through it, what is the resistance of the light bulb?
Answers
Answer:
0.6 ohm
Explanation:
voltage = 1.5 V
current = 2.5 A
resistence = ?
V = IR
1.5 = 2.5 * R
1.5 / 2.5 = r
0.6 = R
therefore resistence is 0.6 ohm
Consider the reaction
2NO(g) + O2(g) = 2NO2(g)
Suppose that at a particular moment during the reaction nitric oxide
(NO) is reacting at the rate of 0.066 M/s. (a) At what rate is NO2
being formed? (b) At what rate is molecular oxygen reacting?
Answers
Answer:
(a) Rate of formation of NO2 is also 0.066M/s
(b) Rate of reaction of O2 gas is 0.033M/s
Explanation:
(a) in one second, according to the equation,
2 moles of NO combines with 2moles of NO2.
Therefore 0.066M NO will still consume 0.066mole NO2.
(b) According to the equation,
2 moles NO consumes 1 mole O2, 0.0666M will consume 0.0333 mole O2
The mass of copper obtained experimentally was 0.872g. calculate the percentage yield of copper
Answers
The theoretical yield is the amount of copper that would be obtained if the reaction proceeded with 100% efficiency, based on the stoichiometry of the reaction and the amount of limiting reagent used.
The percentage yield of copper can be calculated using the formula:
Percentage yield = (actual yield / theoretical yield) x 100%
In this case, the actual yield is given as 0.872 g. The theoretical yield is the amount of copper that would be obtained if the reaction proceeded with 100% efficiency, based on the stoichiometry of the reaction and the amount of limiting reagent used.
In general, the percentage yield is a measure of how efficient a chemical reaction is in producing the desired product. It is calculated by comparing the actual yield obtained in the experiment to the theoretical yield that would be obtained under ideal conditions.
A high percentage yield indicates that the reaction is efficient and that the experimental setup is effective in producing the desired product. A low percentage yield indicates that there are inefficiencies or losses in the reaction, and that improvements may be needed in the experimental setup or reaction conditions.
To know more about the Percentage yield , here
https://brainly.com/question/30139773
#SPJ1
25.00 g of aluminum sulfide and 50.00 g of water react until the limiting reagent is used up: which is the limiting reagent? what is the max mass of hydrogen sulfide that can form?
Answers
The maximum mass of hydrogen sulfide that can form is 17.01 g.
What is Limiting Reagent?
A limiting reagent, also known as a limiting reactant, is the substance that is completely used up in a chemical reaction and limits the amount of product that can be formed. It is the reactant that is present in the smallest stoichiometric amount and, as a result, determines the theoretical yield of the reaction. The other reactants may be present in excess and may not be completely used up.
To determine which reactant is the limiting reagent, we need to calculate the amount of product that each reactant would produce and see which one produces less.
The balanced chemical equation for the reaction between aluminum sulfide and water is:
First, we need to calculate the amount of moles of each reactant:
moles of Al2S3 = mass ÷ molar mass = 25.00 g ÷ 150.16 g/mol = 0.1664 mol
moles of H2O = mass ÷ molar mass = 50.00 g ÷ 18.02 g/mol = 2.776 mol
Next, we need to determine the limiting reagent. From the balanced equation, we can see that one mole of Al2S3 reacts with 6 moles of H2O. Therefore, the amount of H2O required to react with 0.1664 mol of Al2S3 is:
0.1664 mol Al2S3 × 6 mol H2O/mol Al2S3 = 0.9984 mol H2O
Since we have 2.776 mol of H2O available, we have more than enough water to react with the 0.1664 mol of Al2S3. This means that aluminum sulfide is the limiting reagent.
To calculate the maximum mass of hydrogen sulfide that can form, we need to use the amount of moles of Al2S3 as the basis of our calculation. From the balanced equation, we know that 1 mole of Al2S3 produces 3 moles of H2S. Therefore:
moles of H2S = moles of Al2S3 × 3 mol H2S/mol Al2S3 = 0.1664 mol × 3 mol H2S/mol Al2S3 = 0.4992 mol H2S
Finally, we can calculate the mass of hydrogen sulfide produced using its molar mass:
mass of H2S = moles of H2S × molar mass = 0.4992 mol × 34.08 g/mol = 17.01 g
Therefore, the maximum mass of hydrogen sulfide that can form is 17.01 g.
Learn more about Limiting Reagent from given link
https://brainly.com/question/30628347
#SPJ1
I NEED THIS NOW AND NO LINKS OR ILL REPORT
Which material creates the most waste and pollutants when creating one
ton of bottles? *
aluminum
glass
plastic
Answers
Answer:
plastic
........................
if there are more products than reactants, does that mean there is an increase in the forward or backward reaction? And if there are more reactants that products, is there an increase in the forward or backward reaction?
Answers
Answer:
If there are more products than reactants, that means the reaction has shifted towards the left, which is the backward direction. If there are more reactants than products, that means the reaction has shifted towards the right, which is the forward direction.
Sir Isaac Newton's only discovery was the three laws of motion
Answers
Answer:
No.
Explanation:
He also created a telescope and formula for universal gravity.
The two chemicals that were in the 1st unknown solution where...
Sodium Chloride
Potassium Chloride
Barium Chloride
Strontium Chloride
Copper Chloride
Lithium Chloride
Calcium Chloride
Answers
The first undiscovered remedy is barium chloride.
Option b is correct.
When tested in a metals lab using a flame, what color did the potassium solution burn?Explanation and Response: The flame of potassium, often known as a lilac flame, is violet or light purple in color. Remember that different elements can create similar colors, thus flame tests are not always reliable.
What should I avoid with respect to cucl2 and LiCl?Avoid contact with mucous membranes, eyes, and skin as copper(II) chloride is mildly poisonous. Body tissue irritant lithium chloride is used. In order to prevent trashcan fires, rinse the wooden splints before throwing them in the trash. Put on chemical-resistant gloves, an apron, splash goggles, and other protective gear.
To know more about chemicals visit:-
https://brainly.com/question/13145357
#SPJ4
2. How many hydrogen bonds can form between a single ether molecule and water molecules? Draw the structures to explain.
Answers
Ether can only form one hydrogen bond per molecule.
What is hydrogen bonding?We know that the hydrogen bond is the kind of bond that occurs when the dipole of water interacts with the dipole that is on another molecule. We can see this in a lot of hydrides of electronegative elements.
We can see that ether has only one electronegative atom and that is oxygen from the image that is shown in the answer. This oxygen atom can interact with the positive end of the dipole in only one water molecule at a time.
Learn more about hydrogen bonding:https://brainly.com/question/15099999
#SPJ1
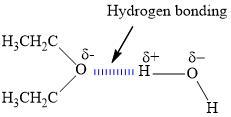
How do molecules of element and molecules of compound differ?what theys are similar?
Answers
According to the research, the molecules of compound are combinations of chemical elements of different complexity, while the molecules of element are made up of atoms of the same type.
What are the molecules of compound and an element?The molecules of compound are a mixture, its elements can have any proportion interrelated by chemical bonds and maintain their properties, while the molecules of element is the set of atoms of the same type that cannot be broken down into simpler ones.
The molecules of compound and an element have a fixed and defined chemical composition, belonging to pure substances, where all the particles, atoms, molecules and/or elements that compose it are exactly the same.
Therefore, we can conclude that according to the research, the molecules of compound are combinations of chemical elements of different complexity, while the molecules of element are made up of atoms of the same type.
Learn more about molecules of compound here: https://brainly.com/question/13054870
#SPJ1
What volume in
L
of a 0.724 M Nal solution contains 0.405 mol of Nal ?
Answers
Answer:
0.559 L
Explanation:
Step 1: Given data
Moles of sodium iodide (n): 0.405 mol
Molar concentration of sodium iodide (M): 0.724 M (0.724 mol/L)
Step 2: Calculate the volume of solution (V)
The molarity is equal to the moles of solute divided by the liters of solution.
M = n/V
V = n/M
V = 0.405 mol/(0.724 mol/L) = 0.559 L
(hope this helps can i plz have brainlist :D hehe)
7.71 Classify each of the following as exothermic or endothermic:
a. CHÂ(g) + 2O₂(g) —^> CO₂(g) + 2H₂O(g) + 802 kJ
b. Ca(OH)₂(s) + 65.3 kJ →→→ CaO(s) + H₂O(l)
c. 2Al(s) + Fe₂O3(s) — Al₂O3(s) + 2Fe(s) + 850 kJ
Answers
A. Heat is released, so the reaction is exothermic.
B. Heat is absorbed, so the reaction is endothermic.
C. Heat is released, so the reaction is exothermic.
Why are a molecule's atoms as far away from each other as they can get?
A) Electronegativity differences force the atoms apart
B) The protons in the nuclei push the atoms apart
C) Forces between electron pairs push the atoms apart
D) Induced dipoles push the atoms away from each other
Answers
Answer:
the answer is a
Explanation:
an electron contains a negative charge and like charges repel each other. Hence, in a atom the force of repulsion between the atoms away from each other. As a result a molecules atoms are as far away from each others as they can get .
A molecule's atoms as far away from each other as they can get because "Forces between electron pairs push the atoms apart".
So, option C is correct one.
Why electrons repel each other?Since, an electron is negative charge species revolve around the nucleus of an atom. So, when two atoms come close to each other in molecules there is repulsion occurs between negative charge electron .
To learn more about electrons here.
https://brainly.com/question/18367541
#SPJ3
The owner of Grizzly Tea Shack is thinking about adding iced tea to the menu. He
thinks he can do this with minimal effort by adding ice cubes to cups of hot tea.
He decides to measure how changing the number of ice cubes in a glass of
freshly brewed tea affects its cooling rate.
To begin, the owner varies the number of ice cubes, x, he puts in glasses of
freshly brewed tea. He then checks the temperature (in Celsius), y, of each glass
after 10 minutes.
Ice cubes Temperature after 10 minutes (in degrees Celsius)
2
17
3
5
6
6
20
10
11
15
Round your answers to the nearest thousandth.

Answers
Answer: 5,266
Explanation:
5,266