Answers
To make a stock solution, weigh out the proper amount of a pure solid or measure out the proper amount of a pure liquid, put it in the right flask, and then dilute it to the desired volume. Depending on the intended concentration unit, many methods can be used to measure the reagent.
If you take a 13.0- mL portion of the stock solution and dilute it to a total volume of 0.650 L , what will be the concentration of the final solution?
"The final solution's concentration would be 0.26M."
By adding extra solvent, you are lowering the concentration of the stock solution in the issue (This process is known as dilution).
Since you increased the volume of the fluid from 13 to 650 milliliters, it was diluted:
50 times = 650 mL / 13 mL
We must now take the concentration of the stock solution in order to determine the concentration of a final solution.
If the standard solution is 13M, then
The final solution's concentration would be 13M / 50 = 0.26M.
To know more about stock solution and final solution, click on the link below:
https://brainly.com/question/20316955
#SPJ9
Related Questions
a. Identify the structures shown in the diagram. b. Identify the information that is contained within these structures. c. Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person. d. Explain why the structures are in pairs.
Answers
The answer responses to the structures shown in the diagram are:
A. chromosomes
C. They would be the same.
B. They are in pairs because each one comes from a different parent.
What is the structure about?The chromosomes are in pairs because humans have a diploid number of chromosomes, meaning they have two sets of chromosomes, one inherited from each parent.
The nucleus is important in eukaryotic cells and has many important parts that help the cell work properly. There are some parts inside cells called the nuclear membrane, nucleoplasm, nucleolus, and chromatin. Chromatin is made up of DNA and other proteins.
Every part of a person's body has the same genes, but the way they are organized can be different in different types of cells. The chromosomes in our skin cells might not be the same as the chromosomes in our muscle cells, even if they come from the same person.
Learn more about nucleus from
https://brainly.com/question/9376695
#SPJ1
Identify the structures shown.
A. chromosomes
B. mitochondria
C. nuclei
D. vacuoles
C
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Explain why the structures are in pairs.
A. They aren't in pairs.
B. They are in pairs because each one comes from a different parent.
C. This cell is making a copy of itself.
D. The cell always has 2 copies in case 1 is damaged.
Complete and balance equation KI(aq)+BaS(aq)→
Answers
The balanced reaction equation is; 2KI(aq)+BaS(aq)→ K2S(aq) + BaI2(aq)
What is equation?A chemical equation shows the conversion of reactants into products. Recall that in a chemical reactions, reactans interact with each other to yield products.
For the reaction we have in the question,the complete reaction equation is; 2KI(aq)+BaS(aq)→ K2S(aq) + BaI2(aq)
Learn more about reaction equation; https://brainly.com/question/16921139
Please help me on this! Please

Answers
Observation vs Inference
An observation is based on factual information that can be found through the use of the 5 senses: touch, taste, smell, sight, hearing. An inference is a guess or conclusion that is made after something is observed.
1. The clouds mean it is going to rain today.
the clouds have been observed by sight and a guess has been made that it will rain as a result of this observation. Therefore it is an Inference.It's rainy and cold.
both of these are factual information based on the 5 senses. Rain can be seen by sight and you can feel whether something is hot or cold, Therefore it is an Observation.The grass is going to get very green after the rain.
The rain can be observed by sight but there is a guess that the grass will become very green. There was an observation and a inference made upon that observation. Therefore it is an Inference.1. What do we achieve by keeping the concentration of sodium hydroxide significantly higher than the crystal violet (CV) concentration
Answers
Answer:
The molar concentration of the crystal violet solution is more concentrated than that of the sodium hydroxide solution. It is because the crystal violet solution has more solute in it compared to the sodium hydroxide.
what is a jump start that can speed up the decomposition reaction in soda
Answers
Answer:
you have to shake the soda up
how many grams of p2o3 can be produced from 95l o2 at STP
Answers
Explanation:
Balanced Equation
P
2
O
3
+
3H
2
O
→
2H
3
PO
3
First use the balanced equation to determine the mole ratio between
H
3
PO
3
and
P
2
O
3
. This ratio will be used to determine the moles
P
2
O
3
required to produce
10.2
moles
H
3
PO
3
.
Mole Ratio Between
P
2
O
3
and
H
3
PO
3
from the balanced equation.
1
mol P
2
O
3
2
mol H
3
PO
3
and
2
mol H
3
PO
3
1
mol P
2
O
3
Multiply the moles
H
3
PO
3
by the molar mass that cancels
H
3
PO
3
and leaves
P
2
O
3
10.2
mol H
3
PO
3
×
1
mol P
2
O
3
2
mol H
3
PO
3
=
5.10 mol P
2
O
3
Now that the moles
P
2
O
3
required to produce
10.2 mol H
3
PO
3
are known, multiply the number of moles by its molar mass,
109.945 g/mol
. https://www.ncbi.nlm.nih.gov/pccompound?term=P2O3 This will give the mass in grams needed for
P
2
O
3
to produce
10.2 mol H
3
PO
3
.
5.10
mol P
2
O
3
×
109.945
g P
2
O
3
1
mol P
2
O
3
=
561 g P
2
O
3
rounded to three significant figures
A unimolecular elementary step involves only a single reactant that breaks apart.a) trueb) false
Answers
Answer:
True
Explanation:
The correct option is; True.
Unimolecular elementary steps in a reaction requires only one reactants, they are usually decomposition reactions. They take the general form;
A --> B + C
You want to go swimming, and the pH of a water in your swimming pool is 7.9. You want it to be between 7.4-7.6. What should you add to the pool to lower its pH?
1. Water
2. Alkali
3. Acid
Answers
Answer:
i think Alkali is a right ans
What is the percent yield for the reaction below when 364 g SO2 and 42.0 g
02 produce 408 g SO3?
2SO₂(g) + O₂(g) → 2SO3(g)
A. 89.7%
B. 97.1%
C. 51.5%
D. 100%
Answers
C. 51.5% is the percent yield for the reaction below when 364 g SO2 and 42.0 g O2 produce 408 g SO3
How is percentage yield calculated?The actual yield is determined by calculating the quantity of the product created. We can estimate the percentage yield by dividing the actual yield by the theoretical value. The percentage yield is the difference between the quantity of product that was actually created and the maximum calculated yield.
2SO₂(g) + O₂(g) → 2SO3(g)
SO₂ mass => 364g
O₂ mass => 42g
SO3 mass => 408g
Theoretical yield of SO3:
mole of O₂ => 42g/32g => 1.31
according to the chemical equation:
1 mole of oxygen => 2 moles of SO3
1.31 mole O2 => x moles of SO3
x=> 2 x 1.31 => 2.62
Hence, the mass of SO3 => 2.62 x 80.06 => 209.75g
The percentage of yield => Actual yield / theoretical yield x 100
=> 209.75/408 x 100 => 51.45 => 51.5%
What differs the theoretical yield from the actual yield?The theoretical yield is the yield that is derived using a balanced chemical reaction. What you actually acquire from a chemical reaction is the actual yield.
Learn more about percentage yield here:
brainly.com/question/29767432
#SPJ1
Question: What volume of 4.50 M HCI can be
made by mixing 5.65 M HCI with 250.0 mL of
3.55 M HCI?
Answers
Approximately 0.157 liters or 157 milliliters of the 4.50 M HCl solution can be made by mixing the given solutions.
To determine the volume of 4.50 M HCl that can be made by mixing the given solutions, we can use the concept of the concentration-volume relationship:
C1V1 = C2V2
Where:
C1 = concentration of the first solution
V1 = volume of the first solution
C2 = concentration of the second solution
V2 = volume of the second solution
Let's assign the variables as follows:
C1 = 5.65 M
V1 = unknown volume (we'll solve for this)
C2 = 3.55 M
V2 = 250.0 mL = 0.250 L (since the volume is given in milliliters)
Now we can plug in the values into the equation and solve for V1:
(5.65 M)(V1) = (3.55 M)(0.250 L)
Dividing both sides of the equation by 5.65 M:
V1 = (3.55 M)(0.250 L) / 5.65 M
V1 ≈ 0.157 L
For more question on solutions click on
https://brainly.com/question/25326161
#SPJ11
Copper metal has a face-centered cubic structure with all atoms at lattice points and a density of 8.93 g/cm^3. The edge length of the unit cell is 361.5 pm. Calculate the mass of 1 atom of copper.
Answers
The mass of 1 atom of copper metal in the given face-centered cubic structure is determined as 1.054 x 10⁻²² g.
Mass of 1 atom of copperThe mass of 1 atom of copper is calculated as follows;
mass of 1 atom of copper = molar mass of copper / Avogadro's number
substitute the value of molar mass of copper and Avogadro's number;
mass of 1 atom of copper = (63.5 g/mol) / (6.023 x 10²³)
mass of 1 atom of copper = 1.054 x 10⁻²² g
Thus, the mass of 1 atom of copper is determined as 1.054 x 10⁻²² g.
Learn more about copper metal here: https://brainly.com/question/24856041
#SPJ1
Which reason best explains why metals are malleable?
A. because they have delocalized electrons
B. because they have localized electrons
C. because they have ionic bonds
D. because they have rigid bonds
The correct answer is A on edg 2020
Answers
Why is the cutting edge of a knife made very thin?
Answers
Answer:
A sharp knife cut objects better because due to its very thin edge, the force of our hand falls over a very small area of the object producing a large pressure and this large pressure cuts the object easily.
Solid manganese(IV) oxide reacts with the solid aluminum metal to produce solid manganese and solid aluminum oxide. Balance the equation for this reaction (in the lowest multiple integers). What are the formulas for the reactants and products?

Answers
1. Balanced equation of the reaction = \(3MnO_2(s) + 4Al (s) -- > 2Al_2O_3(s) + 3Mn(s)\)
2. Formulas of reactants
solid manganese (IV) oxide = \(MnO_2 (s)\)
solid aluminum metal = \(Al(s)\)
solid manganese = \(Mn (s)\)
solid aluminum oxide = \(Al_2O_3 (s)\)
Balancing chemical equationsA balanced chemical equation agrees with the law of conservation. In other words, the number of participating atoms must balance between the reactants and the products of the same equation.
Formula for solid manganese (IV) oxide = \(MnO_2 (s)\)
Formula for solid aluminum metal = \(Al(s)\)
Formula for solid manganese = \(Mn (s)\)
Formula for solid aluminum oxide = \(Al_2O_3 (s)\)
Thus, the balanced equation for the reaction is written as:
\(3MnO_2(s) + 4Al (s) -- > 2Al_2O_3(s) + 3Mn(s)\)
One can see from the that the number of atoms of each element in the reactants is equal to the number of atoms of the same elements in the product.
More on balancing chemical equations can be found here: https://brainly.com/question/28294176
#SPJ1
Reactions
Predict the products for the single replacement reactions given. Check to see that the equations are
balanced.
Ca + MgCl2 →?
-
O CaCl2 + Mg
O Ca + Cl2
0 Mg + Cl2
CaCl + Mg
Answers
Answer:
answer in picture
Explanation:

The balanced chemical equation will be Ca + MgCl2 → CaCl2 + Mg.
What is balanced chemical equation?An equation that has the same quantity of atoms on both sides of the arrow would be said to represent a balanced chemical reaction.
The given equation is Ca + MgCl2 →
It is known that when Ca is reacted with magnesium chloride ( MgCl2) then it will form calcium chloride (CaCl2 ) and magnesium.
Ca + MgCl2 → CaCl2 + Mg.
It can be seen that, calcium has one atom in the reactant side and one atom in product side. On the other hand, chlorine has one atom in reactant side as well as product side.
Hence, the reaction will be balanced.
To know more about balanced chemical reaction.
https://brainly.com/question/15052184
#SPJ3
There are three isotopes of X element (X):
X-17 isotope: atomic mass17.2 amu, abundance:78.99%
X-18isotope: atomic mass 18.1 amu, abundance 10.00%
X-19isotope: atomic mass:19.1 amu, abundance: 11.01%
Calculate the average atomic mass of X.
Answers
Answer:
Average atomic mass = 17.5 amu.
Explanation:
Given data:
X-17 isotope = atomic mass17.2 amu, abundance:78.99%
X-18isotope = atomic mass 18.1 amu, abundance 10.00%
X-19isotope = atomic mass:19.1 amu, abundance: 11.01%
Average atomic mass of X = ?
Solution:
Average atomic mass = (abundance of 1st isotope × its atomic mass) +(abundance of 2nd isotope × its atomic mass) + (abundance of 3rd isotope × its atomic mass) / 100
Average atomic mass = (78.99×17.2)+(10.00×18.1) +(11.01+ 19.1) /100
Average atomic mass = 1358.628 + 181 +210.291 / 100
Average atomic mass = 1749.919 / 100
Average atomic mass = 17.5 amu.
draw a diagram showing the electronic configuration of chlorine and the composition of the nucleus of the chlorine atom
Answers
The electronic configuration of the chlorine atom can be written as 2, 8 , 7
What is electron configuration?The positioning of electrons within an atom's or molecule's orbitals is referred to as its electronic configuration. Around the atomic nucleus of an atom, electrons—negatively charged subatomic particles—occupy various energy levels or shells.
The electrons in an atom fill these energy levels or shells in a certain order in accordance with a series of laws known as the Aufbau principle, Pauli exclusion principle, and Hund's rule. Each energy level or shell has a maximum capacity for electrons.
Learn more about electron configuration:https://brainly.com/question/29757010
#SPJ1

The combination of a heart,arteries,veins,and capillaries is
A. CELL
B. an organ system
C. organism
D. tissue
PLEASE HELP ME FAST!!!!!!
Answers
Answer: B
Explanation:
which probing question lies within the scope of physics?
Answers
Physics is a vast field that addresses a wide range of questions about the nature of the physical world. Probing questions can help to explore this field and encourage critical thinking and deep exploration of its topics.
Probing questions are open-ended questions asked to gather information, encourage critical thinking and deep exploration of a particular topic. Physics is a natural science that studies matter and its motion through space-time. It is a branch of science that deals with the fundamental nature of the universe and seeks to explain how and why objects behave as they do in the physical world.The following are some examples of probing questions within the scope of physics:What is the nature of light-The nature of light is an important topic within the scope of physics. It refers to the dual nature of light, as both a wave and a particle. Light behaves as a wave when it is traveling through space and as a particle when it is interacting with matter.How do magnets work-Magnets are a common object in the world around us, and they have a broad range of applications. They work by producing a magnetic field, which can attract or repel other magnetic objects. This topic lies within the scope of physics.What is the relationship between energy and matter-Energy and matter are two fundamental concepts in physics. The relationship between them is described by Einstein's famous equation E=mc2, which states that matter and energy are two forms of the same thing and are interchangeable. The study of the relationship between energy and matter lies within the scope of physics.What is the nature of the universe?The study of the universe's nature is one of the most significant topics within the scope of physics. This question addresses the origins and properties of the universe, its components, and the laws that govern its behavior.
for such more questions on physical
https://brainly.com/question/1079154
#SPJ8
Rank the following gases in order of increasing solubility in ammonia (NH3) where 1 is
the least soluble and 4 is the most soluble.
SO2 (sulfur dioxide), N₂ (nitrogen), H₂O (water), H₂ (hydrogen)
Answers
Water (H2O) is more soluble in Ammonia (most soluble)
Nitrogen gas then follows as the next. (soluble)
Sulphur (iv) oxide is the least soluble in Ammonia.(least soluble)
“surely you have gotten into a car on a cool day and noticed that the metal buckle on your seatbelt feels significantly colder than other things in your car, such as the steering wheel or the center console. Explain why this is” someone please help
Answers
Answer:
The metal buckle conducts heat away from the hand than both the steering wheel and center console.
Explanation:
Good conductors are materials that allow both heat and electricity to flow easily through them. Examples of good conductors are metals.
Poor conductors or insulators are materials which do not allow both heat and electricity to flow easily through them. Examples of insulators are most non-metals, plastics, rubber, wool, wood, etc.
On a cool day a metallic object like the buckle of a car's seatbelt will easily conduct heat away from the hand than other materials inside the car like the seat, the seatbelt, the steering wheel and the centre console as these materials are made from insulators or poor conductors.
Therefore, the metal buckle will feel colder to touch than other things in the car because it conducts heat away from the hand than both the steering wheel and center console.
Place the following compounds in order of decreasing strength of intermolecular forces. I. CH3CH2CH2CH2CH2CH3 II. (CH3)3CCH3 IIL (CH3)3CCH2CH3 1) C) II >III> I 2) Identify the compound with the lowest boiling point. A) CH3CN B) CH3CHO C) CH3CH2CH3 D) (CH3)20 E) CH3OCH3 2) 3) Place the following substances in order of increasing vapor pressure at a given temperature SF6 SiH4 SF4 3) A) SiH4
Answers
The following compounds in order of decreasing strength of intermolecular forces is as follows :
CH₃CH₂CH₂CH₂CH₂CH₃ > (CH₃)₃CCH₂CH₃ > (CH₃)₃CCH₃
1) The following compounds in order of decreasing strength of intermolecular forces is as follows : as the surface are decreases the intermolecular force decrease or as branching increases intermolecular forces decreases.
CH₃CH₂CH₂CH₂CH₂CH₃ > (CH₃)₃CCH₂CH₃ > (CH₃)₃CCH₃
2) the compound CH₃CH₂CH₃ have the weaker van der wall forces and having the lowest boiling point.
3) the following substances in order of increasing vapor pressure at a given temperature is as follows :
SF₄ < SF₆ < SiH₄
SF₄ is the polar molecular and hs the lowest vapor pressure.
To learn more about intermolecular force here
https://brainly.com/question/14774937
#SPJ4
Do you think it's possible for someone to completely eliminate procrastination from their life? Why or why not
Answers
Answer:
No and Yes
Explanation:
This is both possible and impossible. When you put your mind to what you believe, you can achieve it through patience and minded values. Your mind can either be your Greatest enemy or your Best Ally.
here is paragraph 2 and i need help pls..lmk if u need more info


Answers
Answer:
i would say the first one!
Help help help !!!!!!
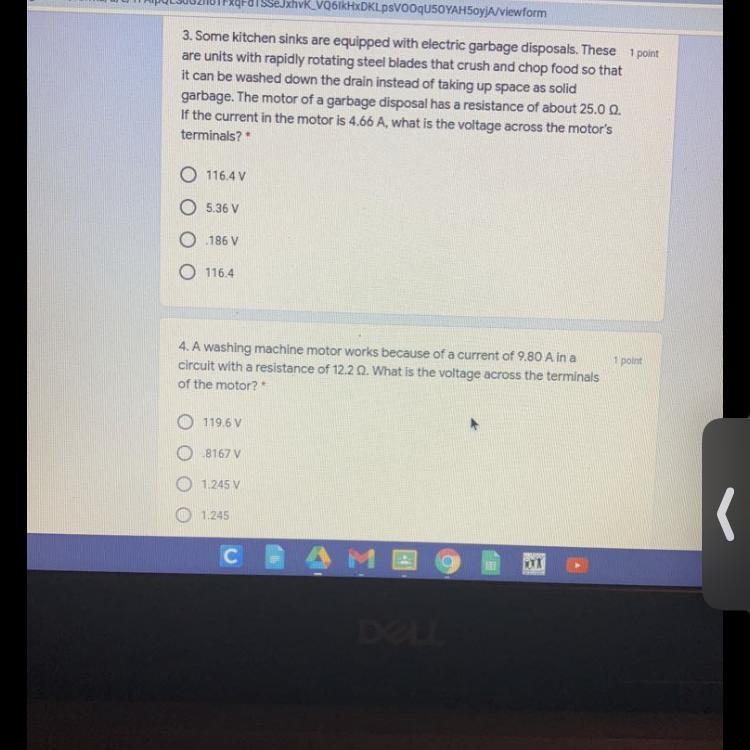
Answers
Answer:
3. 116.5 V
4. 119.6 V
Explanation:
3. Determination of the voltage.
Resistance (R) = 25 Ω
Current (I) = 4.66 A
Voltage (V) =?
V = IR
V = 4.66 × 25
V = 116.5 V
Thus, the voltage is 116.5 V
4. Determination of the voltage.
Current (I) = 9.80 A
Resistance (R) = 12.2 Ω
Voltage (V) =?
V = IR
V = 9.80 × 12.2
V = 119.6 V
Thus, the voltage is 119.6 V
explain in details what a matter is...!!!
Answers
Answer:
umm
Explanation:
matter makes up everything. everything is matter
the more matter an object has the more mass It has
PLEASEEEEE HELP
1 Explain why decan-1-ol CH,(CH,) CH,OH has
only limited solubility in water, whereas ethanol
CH,CH,OH is miscible with water in all proportions.
2 Give details of the reagents and the practical
procedures required to prepare each of the following
substances from a named alcohol:
a 1-Chloropropane
b 2-Bromopropane.
3 Explain why sodium metal reacts more slowly with
ethanol CH,CH,OH than with water HOH.
4 Draw Lewis structures to show the formation of the
ethoxide ion by the action of sodium on ethanol.
Answers
Answer:
Can you arrange it? I don't understand
A carbon atom has a relative atomic mass of
13.3. 60% of the carbon atom has a mass
number of 12.7, 30% of the carbon atom has a
mass number of 13.6, whilst the remaining 10%
has a mass number which is not known.
Calculate the mass number of the carbon isotope
which is 10% of the Carbon 13.3 atom.
Give your answer to 1 decimal place.
Answers
According to the claim, the carbon isotope that is 10% of something like the carbon 13.3 molecule has a mass number of 15.1.
Where can you find carbon?The majority of carbon is kept in reservoirs, or sources, such rocks and sediments; the remaining portion is kept in the atmosphere, the seas, and living things. Through the respiration of both and animals, carbon is returned to the atmosphere. Burning things like wood, oil, and gas also releases it.
Briefing:Since all carbon isotopes have an equal atomic mass of 13.3,
(0.60 * 13.3) + (0.3 * 12.7) + (0.1 * x) = 13.3
11.79 + 0.1x = 13.3
x = 15.1
To know more about Carbon visit:
https://brainly.com/question/13719781
#SPJ1
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
Which type of covalent bond is the least stable?
A.Covalent bonds
B.Double bonds
C.Single bonds
D.Triple Bonds
Answers
Answer:
D because: A Triple bond is when three pairs of electrons are shared between two atoms in a molecule. It is the least stable out of the three general types of covalent bonds.