Answers
If you increase the pressure of the reaction 2A(g) + B(g)C(g) + 2D(g)the reaction will shift forward to the side with little moles of gas.
What is the reaction about?Le Chatelier's principle states that if pressure is raised, the reaction will favor the direction with a lower number of gas moles.
On this case, the cleared out side includes a add up to of three gas moles (two moles of A and one mole of B), whereas the correct side covers four gas moles (one mole of C and two moles of D). In this way, the balance will move towards the left-hand side in arrange to diminish the collective gas particles and relieve the rise in weight. The levels of A and B will rise, as the levels of C and D will decrease.
Learn more about pressure from
https://brainly.com/question/28012687
#SPJ1
Related Questions
Select the atoms whose ions have an electron configuration of nd^6 (n = 3, 4, 5 ...). For example, the 2+ ion for V ends the electron configuration with 3d^3 .Check all that apply.A. Tl B. Fe C. Rh D. La E. Os F. Ti
Answers
Answer
Correct answers are B. Fe and E. Os
Explanation
For Fe: electron configuration for Fe is
\([Ar]3d^64s^2\)For TI: the electron configuration is
\([Xe]4f^{14}5d^{10}6s^26p^1\)For Rh: Electron configuration of Rhodium is
\([Kr]4d^85s^1\)For La: Electron configuration of Lanthanum is
\([Xe]5d^16s^2\)For Os: Electron configuration is
\([Xe]4f^{14}5d^66s^1\)For Ti: Electron configuration is
\([Ar]3d^24s^2\)Which option describes a phase change?
OA. Plasma erupting from the sun
DB. A helium balloon rising into the sky
OC. Gasoline evaporating into the air
Air leaking from a tir
Answers
I apologize of I'm wrong but its probably OA
Sorry once again!
~wise rat
Answer: C.
Explanation:
gasoline evaporating into the air
100 POINTS!!!
What is the average rate of the reaction over the entire course of the reaction?
1.6 × 10−3 (?)
1.9 × 10−3 (?)
2.0 × 10−3 (X)
2.2 × 10−3 (X)

Answers
Answer:
b. 1.9 × 10-3
Explanation:
Answer:1.9x10-3
Explanation:
average
What would happen if two negative charges were next to each other? What if a positive charge and a negative change were next to each other?
Answers
Answer:
if two negative charges are next to each other they repel.
But if positive and negative charges are next to each other they attract.
Explanation:
Why because like changes repel and unlike charges attract
A rectangular metal is 25 dm long, 525 mm wide, and 1.5 m high. This metal weighs 250 lb. Calculate its density in g /mL
Answers
Answer:
d = 0.057 g/mL
Explanation:
Given that,
The dimensions of a rectangular metal is 25 dm long, 525 mm wide, and 1.5 m high.
The weight of the metal is 250 lb
We need to find the density of the metal in g/mL.
1 lb = 453.592 g
250 lb = 113398 g
Volume of the block,
V = 25 dm× 525 mm×1.5 m
Since, 1 dm = 0.1 m, 1mm = 0.001 m
V = 2.5 m× 0.525 m× 1.5 m
V = 1.9668 m³
Also, 1 m³ = 1968000 mL
So,
density = mass/volume
density = 113398 g/1968000 mL
= 0.057 g/mL
So, the density of a rectangular metal is 0.057 g/mL.
4.
Ethanol has a specific heat of 2.44 (J/g.°C). The temperature of 34.4 g of ethanol increases
from 25 °C to 78.8 °C. How much Heat was absorbed?
Answers
how many grams of carbon dioxide are formed when 100.00g of propane (C3H8) is burned
Answers
100.00g of propylene (C3H8) burns to produce 6.82 mol of dioxide.
Produces CO2 when propane is burned?When propane is burned completely, carbon dioxide and water vapor are produced. When there is not enough oxygen present for the propane to completely burn, carbon monoxide is produced as a byproduct of combustion.
How are burn percentages determined?It is determined by deducting operational costs from revenue. Additionally, it is evaluated monthly. It demonstrates how much money a business requires to stay afloat for a while. We are aware that burning propane or other hydrocarbons results in a biochemical reaction because dioxide and water purification products are produced.
To know more about carbon dioxide visit:
https://brainly.com/question/3049557
#SPJ1
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale). If this is true, what determines the difference between a slate and a gneiss rock that both are formed from shale? What role does the parent rock play in determining the type of metamorphic rock that will be formed?
Answers
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale) is a true statement.
The parent rock, in this case shale, plays a significant role in determining the type of metamorphic rock that will be formed. The minerals and structure of the parent rock provide the starting material for the metamorphic rock, and the specific conditions under which the rock undergoes metamorphism determine the final characteristics of the metamorphic rock.What determines the difference between a slate and a gneiss rock that both are formed from shale?Slate, phyllite, schist, and gneiss are all types of metamorphic rocks that can be formed from shale, which is a sedimentary rock composed of clay and other fine-grained minerals. The specific type of metamorphic rock that is formed from shale depends on the conditions under which the shale undergoes metamorphism, including the temperature, pressure, and presence of fluids.
Slate is a fine-grained metamorphic rock with a uniform, flat surface and a layered structure. It is formed when shale undergoes low-grade metamorphism, which occurs at relatively low temperatures and pressures.
Therefore, Gneiss, on the other hand, is a medium- to coarse-grained metamorphic rock with a banded or wavy texture. It is formed when shale undergoes high-grade metamorphism, which occurs at higher temperatures and pressures.
Learn more about Metamorphic Rock from
https://brainly.com/question/1176274
#SPJ1
Where would you find the asthenosphere?
A. layer
B. upper mantle
C. Moho Discontinuity
Answers
Answer:
b. upper mantle
Explanation:
low velocity zone of the upper mantle
Can someone please answer this.

Answers
All matter can be classified as two main types of materials. Identify below 6 points whether each of these is one of the two main types of matter.

Answers
Answer:c
Explanation:
Br2(g) + 3 F2(g) ⇄ 2 BrF3(g) Kp = 5.4 × 108 0.30 atm of Br2 and 0.60 atm of F2 are placed in a 3.0 L container and the system is allowed to reach equilibrium. Calculate the pressureof Br2 at equilibrium.
Answers
Answer:
The pressure at equilibrium of Br₂ is 0.10048 atm
Explanation:
Based on the reaction:
Br₂(g) + 3 F₂(g) ⇄ 2 BrF₃(g)
Kp is defined as:
\(Kp = \frac{P_{BrF_3}^2}{P_{Br_2}P_{F_2}^3}\) = 5.4x10⁸
If initial pressures of Br₂ and F₂ are 0.30atm and 0.60atm respectively, the pressures in equilibrium are:
Br₂ = 0.30atm - X
F₂ = 0.60atm - 3X
BrF₃ = 2X
Replacing in Kp formula:
5.4x10⁸ = [2X]² / [0.30atm - X] [0.60atm - 3X]³
5.4x10⁸ = 4X² / [0.30 - X] [0.216 - 3.24 X + 16.2 X² - 27 X³]
5.4x10⁸ = 4X² / 0.0648 - 1.188 X + 8.1 X² - 24.3 X³ + 27 X⁴
Solving for X:
X = 0.3000 → False answer because produce negative concentrations.
X = 0.19952. Replacing in equation of Br₂:
Br₂ = 0.30atm - 0.19952atm = 0.10048 atm
Which is an example of poor safety practices when working outdoors

Answers
Answer:
C
Explanation:
u can't touch a chemical with bare skin
Answer:
touching a chemical with his or her bare skin.
An electrolysis reaction is
A) spontaneous
B) exothermic
C) non-spontaneous
D) hydrophobic
Answers
Answer:
it's non-spontaneous
Explanation:
I hope it helps you
Solid magnesium and chlorine gas react to form solid magnesium chloride. The reaction is exothermic. Which diagram best represents this reaction?
Answers
Answer:
see the pic for more detail

Show the relationship of number of moles and volume in Avogadro's law using data. Write your calculations and answer on a separate sheet of paper. Plot these values in hypothetical the volume-mole graph below.
Guide Questions:
1. What do you notice on the calculated values of v/n?
2. How does your volume -mole graph look like?
PLEASE HELP ME

Answers
Answer:
See below ~
Explanation:
The calculated values of V/n :
⇒ 1.5/0.3 = 5
⇒ 3/0.6 = 5
⇒ 4.5/0.9 = 5
⇒ 6/1.2 = 5
⇒ 7.5/1.5 = 5
1. From this we understand that the calculated values of V/n remain constant, equal to 5 in this case.
2. The volume-mole graph will be a straight line passing through the origin. (Attached below)

what is the weight in newton’s of a person with a mass of 80 kg
Answers
Answer:784.532 newton
What would be the final value for the enthalpy CO2+2h2o h =-1410 Kj
Answers
The final value for the enthalpy change of the formation of CO2 and 2H2O from their elements (C, H2, and O2) would be -1410 kJ per mole of CO2 and 2 moles of H2O formed.
The enthalpy change (ΔH) for the reaction CO2 + 2H2O → H2CO3 can be calculated by multiplying the stoichiometric coefficients of the balanced equation by the enthalpy values of the corresponding compounds involved in the reaction.
In the given reaction, the enthalpy change is -1410 kJ. However, it's important to note that this enthalpy change corresponds to a specific reaction and may not directly apply to the formation of CO2 and 2H2O from another reaction or process.
If we assume that the reaction is the formation of one mole of CO2 and two moles of H2O, we can say that the enthalpy change for this specific formation reaction is -1410 kJ.
Therefore, the final value for the enthalpy change of the formation of CO2 and 2H2O from their elements (C, H2, and O2) would be -1410 kJ per mole of CO2 and 2 moles of H2O formed.
It's worth mentioning that the enthalpy change can vary depending on the specific conditions (temperature, pressure, etc.) and the reactants involved in the reaction. Therefore, it's crucial to specify the conditions and reaction context when referring to enthalpy values.
For more such questions on enthalpy change visit:
https://brainly.com/question/15174388
#SPJ8
Hydrogen reacts with oxygen according to the balanced equation
2H₂ (g) + O2(g) → 2H₂O(g). If X is the number of molecules of H₂ which react,
then the number of O2 molecules reacting is
Answers
Answer:
x/2
Explanation:
X = 2 molecules of H2
For 2 molecules of H2, there's only 1 molecule of O2. Meaning, there's twice the amount of H2, so O2 = x/2 molecules.
I hope I'm understanding this question right.
which shows the correct order of stelps during the formation of an ionic bond
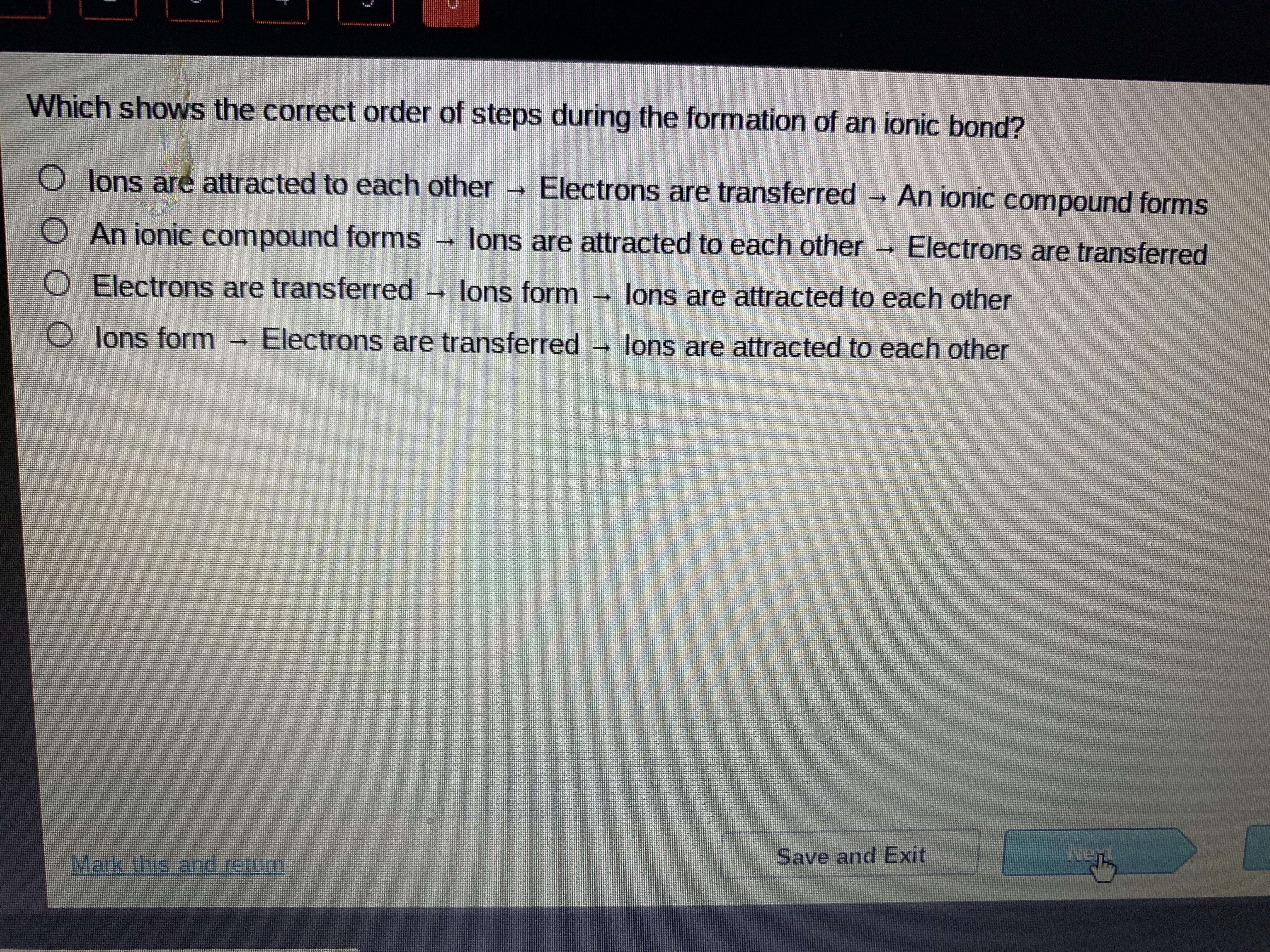
Answers
Answer:
The third one
Explanation:
on food webs can i get some help with that
Answers
A small amount of chemical splashes in Frank’s eye. What should Frank do immediately?
Answers
Answer:
A small amount of chemical splashes in Frank's eye. What should happen next? Frank should go to the eyewash station while his lab partner tells the teacher what happened.
Explanation:
Brainlist
How much NaAlO2 (sodium aluminate) is required to produce 2.59 kg of Na3AlF6?
Answers
839 g is the mass of sodium aluminate that is required to produce 2.59 kg of Na₃AlF₆.
A body's mass is an inherent quality. Prior to the discoveries of the atom as well as particle physics, it was widely considered to be tied to the amount of material in a physical body.
It was discovered that, despite having the same quantity of matter in theory, different atoms and elementary particles have varied masses. There are various conceptions of mass in contemporary physics that are theoretically different but physically equivalent.
Moles of Na₃AlF₆ = 2150 × 1/209.94
Moles of Na₃AlF₆ = 10.24 mol Na₃AlF₆
The molar ratio is 1 mol NaAlO₂:1 mol Na₃AlF₆
Moles of NaAlO₂ = 10.24 × 1/1 = 10.24 mol NaAlO₂
Mass of NaAlO₂ = 10.24 × 81.97
Mass of NaAlO₂ = 839 g
To know more about mass, here:
https://brainly.com/question/19694949
#SPJ1
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
Look at the diagram of a fuel cell below. A fuel cell with 2 vertical objects labeled A and B connected by an electrical wire through a circle with a M in it. There is an area between the two vertical objects labeled A, and substances flowing to, along, and away from the vertical objects and to the left and right. Which statement describes how electrons move if oxidation occurs on the left side of the cell and reduction occurs on the right side? Electrons move from left to right through Electrons move from right to left through A. Electrons move from left to right through M. Electrons move from right to left through M.
Answers
The electrons move from left to right through the circle labeled "M" to reach the cathode, where reduction takes place.
If oxidation occurs on the left side of the fuel cell and reduction occurs on the right side, the movement of electrons can be described as follows: Electrons move from left to right through the circle labeled "M."
In a fuel cell, the process of oxidation takes place at the anode (labeled A) where the fuel is oxidized, releasing electrons. These electrons then flow through the external electrical circuit, represented by the wire connecting objects A and B. The electrons reach the cathode (also labeled A) on the right side of the cell, where reduction occurs.The circle labeled "M" represents the membrane or electrolyte in the fuel cell. This membrane allows the transport of ions but blocks the movement of electrons. As a result, electrons cannot flow directly through the electrolyte but must travel through the external circuit.
This movement of electrons through the external circuit is what generates an electric current that can be used to power electrical devices or systems.
for such more questions on reduction
https://brainly.com/question/21851295
#SPJ8
Examine the chemical equation and statement.
Mg+2HCl⇌MgCl2+H2
The reaction is at equilibrium. The forward reaction is endothermic, and the reverse reaction is exothermic.
Given a decrease in temperature, how would the reaction shift?
a. A decrease in temperature will cause the reaction to shift toward the endothermic reaction, which has the reactants MgCl2 and H2.
b. A decrease in temperature will cause the reaction to shift toward the endothermic reaction, which has the reactants Mg and HCl.
c. A decrease in temperature will cause the reaction to shift toward the exothermic reaction, which has the reactants Mg and HCl.
d. A decrease in temperature will cause the reaction to shift toward the exothermic reaction, which has the reactants MgCl2 and H2.
Answers
Answer:
d
Explanation:
the endothermic reaction absorbs heat and exothermic emits heat. If we think of this from increasing the temperature, exothermic already emits heat so it would favor an endothermic reaction. In this instance, a decrease in temperature would do the opposite, which would favor the exothermic reaction.
9. The hydrogen concentration of a solution is 5 x 10-8. What is the pH?
Answers
Answer:
pH = 7.30
Explanation:
You can find the pH using the following equation:
pH = -log[H⁺]
In this equation, [H⁺] represents the hydrogen ion concentration. You can plug the given value into the equation and solve to find the pH.
pH = -log[H⁺]
pH = -log[5 x 10⁻⁸]
pH = 7.30
PLEASE HELP ME I NEED TO SUBMIT RIGHT NOW
An isotope has more or less ____________________________ in the nucleus.
Answers
Answer: number of protons?
Or different atomic weight.
I DONT KNOW ;';
A hot metal plate at 150°C has been placed in air at room temperature. Which event would most likely take place
over the next few minutes?
Molecules in both the metal and the surrounding air will start moving at lower speeds.
Molecules in both the metal and the surrounding air will start moving at higher speeds.
The air molecules that are surrounding the metal will slow down, and the molecules in the metal will speed up.
The air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down.
Answers
Answer:
molecules will speed up
Explanation:
The definition of a limiting reactant can be summarized as:

Answers
Answer:
Explanation:
Here, we want to get the correct answer choice
The limiting reactant is the reactant that gets used up and that dictates the amount of products we can have
Take for example, we want to build a car, and we have 5 steering wheels but 100 tyres
A car needs 1 steering wheel and 4 tyres. Thus, the number of steering wheels will determine the number of possible cars produced
The steering wheel is thus the limiting reactant
The correct answer choice is that the reactant that is usede up is the limiting reactant