Hydrogen chloride gas (hcl) diffuses 1.8 times faster than an unknown gas. determine the molar mass of the unknown gas.
Answers
The molar mass of the unknown gas is approximately 11.25 g/mol.
To determine the molar mass of the unknown gas, we can use Graham's Law of Diffusion.
Graham's law states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass In other words:
Rate of diffusion of gas A / Rate of diffusion of gas B = sqrt(Molar mass of gas B / Molar mass of gas A)
Using the given information, we can set up an equation:
1.8 (rate of diffusion of unknown gas) / 1 (rate of diffusion of HCl) = sqrt(Molar mass of HCl / Molar mass of unknown gas)
Squaring both sides of the equation, we get:
3.24 = Molar mass of HCl / Molar mass of unknown gas
Multiplying both sides by the molar mass of the unknown gas, we get:
Molar mass of unknown gas = Molar mass of HCl / 3.24
The molar mass of HCl is 36.46 g/mol. Plugging this in, we get:
Molar mass of unknown gas = 36.46 g/mol / 3.24
Molar mass of unknown gas = 11.25 g/mol (rounded to two decimal places)
Therefore, the molar mass of the unknown gas is approximately 11.25 g/mol.
Know more about Graham's Law here:
https://brainly.com/question/31488584
#SPJ11
Related Questions
With regard to tonicity, two solutions that have the same concentrations of nondiffusible solutes and water are said to be?
Answers
Any external solution that contains the same amounts of solutes or water as biological fluids is seen to be isotonic. The flow of water inside an isotonic solution won't be net.
Which fatty acids are present in nature Mcq most frequently?The 16- or 18-carbon fatty acids, also known as palmitic acid or stearic acid, respectively, are among the most widely dispersed fatty acids. The bulk of organisms' lipids contain both and stearic acids. In animals, up to 30% of body fat is made up of palmitic acid.
What is the fatty acid's Mcq solubility in water?Water only partially dissolves fatty acids. The hydrophilic COOH group just at end of the hydrocarbon chain is what causes the partial solubility. The hydrocarbon chains cannot be dissolved at all in water.
To know more about solution visit:
https://brainly.com/question/7932885
How many moles of H₂O can be produced when 5.5 moles of CO₂ is produced? C₂H₄ + 3 O₂ --> 2 CO₂ + 2 H₂O
Answers
Answer:
5.5 mol H2O
Explanation:
Given over what you need and then mole ratio of coefficients in the balanced equation then cross multiply and do PEMDAS for that, and then you got your answer :) hope this helped.

1. (04.01 LC)
Which of the following is an example of how science can solve social problems? (5 po
It cathstop excessive rain from occurring.
It can identify the sources of polluted water.
It can control the time and day when cyclones happen.
It can reduce the frequency of severe weather conditions.
2. (04.01 LC)
Answers
Science is used to stop things the are incrediblu diffucult to deal with therefore jeg spiser ikke dreng
The answer is
It can identify the sources of polluted water.
7. convert 22.7g to μg
Answers
The 22.7 g in μg ( micro gram ) is 22.7 × \(10^{-6}\) .
We need to convert between units in order to ensure accuracy and prevent measurement misinterpretation.
For example , we do not measure a pencil's length in kilometers . In this situation , one must convert from kilometers ( km ) to centimeters ( cm ) . In most cases, multiplicative conversion factors are used to convert one unit to another of the same quantity .
Sometimes the units of measurement used may not correspond to the standards required for a particular process or application, as well as the measuring choice and convenience. The mass of object in micro gram unit is less than gram .
to learn more about μg please click here ,
https://brainly.com/question/16630356
#SPJ1
What is technology?
A. The steps that engineers go through to create a product.
B. An understanding of something new.
C. Something created using science for use by society.
D. A method that is used to solve problems.
SUBMIT
Answers
Hypochlorous acid, HOCl is a weak acid commonly used as a bleaching agent. The acid-dissociation constant, Ka, for the reaction of HOCl with water is 3.2*10E-8.
(A) Calculate the (H3O+) of a .14 molar solution of HOCL.
(B) Write the correctly balanced net ionic equation for the reaction the occurs NaOCl is dissolved in water and calculate the numerical value of equilibrium constant for this reaction.
(C) Calculate the pH of a solution made by combining 40.0 milliliters of .14-molar HOCl and 5.0 milliliters of .56-molar NaOH.
(D) How many grams of solid NaOCl must be added to 50.0 milliliters of .20-molar HOCl to obtain a buffer solution that has a pH of 7.49? Assume that the addition of the solid NaOCl results in a negligible change in volumes.
Answers
(A) The (H3O+) of a .14 molar solution of HOCL is 1.20 x 10^-5 M.
(B) The net ionic equation for the reaction of NaOCl with water is: NaOCl + H2O → HOCl + Na+ + OH-. The equilibrium constant for this reaction can be calculated using the acid dissociation constant (Ka) for HOCl and the autoionization constant (Kw) for water: Keq = (Ka/[OH-]) = 2.9 x 10^7.
(C) The pH of the solution made by combining 40.0 milliliters of .14-molar HOCl and 5.0 milliliters of .56-molar NaOH is 8.74.
(D) 8.16 grams of solid NaOCl must be added to 50.0 milliliters of .20-molar HOCl to obtain a buffer solution that has a pH of 7.49.
(A) The first step is to set up the equation for the dissociation of HOCl:
HOCl + H2O ⇌ H3O+ + OCl-
The acid dissociation constant (Ka) is given as 3.2 x 10^-8.
Using the equation for Ka, we can solve for [H3O+]:
Ka = [H3O+][OCl-]/[HOCl]
[H3O+] = √(Ka x [HOCl]) = 1.20 x 10^-5 M.
(B) The balanced net ionic equation for the reaction of NaOCl with water is: NaOCl + H2O → HOCl + Na+ + OH-. The equilibrium constant expression can be written as:
Keq = [HOCl][Na+][OH-]/[NaOCl][H2O]
Since water is in excess, we can assume that its concentration is constant and can be omitted from the equation. Also, assuming complete dissociation of NaOCl, we can write [Na+] = [OCl-]. Thus,
Keq = [HOCl][OH-]/[NaOCl]
Using the acid dissociation constant (Ka) for HOCl, we can write [HOCl][OH-] = Ka x [OCl-].
Substituting this in the Keq expression, we get:
Keq = (Ka/[OH-]) = 2.9 x 10^7.
(C) The balanced equation for the reaction of HOCl with NaOH is:
HOCl + NaOH → NaOCl + H2O
This is a neutralization reaction between an acid and a base.
First, we calculate the moles of HOCl and NaOH used:
Moles of HOCl = 0.14 M x 0.040 L = 0.0056 mol
Moles of NaOH = 0.56 M x 0.0050 L = 0.0028 mol
The limiting reagent is NaOH, which reacts completely with the available HOCl.
The moles of HOCl that remain unreacted = 0.0056 - 0.0028 = 0.0028 mol
The concentration of the remaining HOCl in the final solution = 0.0028 mol/0.045 L = 0.0622 M.
Using the equation for Ka and [H3O+] = √(Ka x [HOCl]), we can calculate the pH of the final solution as 8.74.
(D)The pH of the solution made by combining 40.0 milliliters of .14-molar HOCl and 5.0 milliliters of .56-molar NaOH is 8.74.
For more questions like Reaction click the link below:
https://brainly.com/question/30086875
#SPJ11
A skydiver is in a plane flying at a constant velocity. If the skydiver is trying to land on a
target on the ground, should he jump from the plane when he is directly over the target?
Why or why not?
Answers
Answer:
No,it is not possible to jump directly because the upper body parts are In motion but legparts directly comes in rest due to unbalanced inertia he may get hurt so..
The 500 cubic centimeter of 0.250 M Na2SO4 solution,
added to an aqueous solution of 15.00 grams of barium
chloride, resulted in the formation of a white precipitate of
barium sulfate. How many moles and how many grams of
barium sulfate are formed, respectively?

Answers
Answer:
0.072 moles, 16.776g.
Explanation:
The reaction of Na2SO4 and BaCl2 occurs as follows:
Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + 2NaCl(aq)
To solve this question we must find the moles of each reactant. As the reaction is 1:1, the reactant with the low number of moles is limiting reactant. The moles of limiting reactant = Moles BaSO4. The mass can be obtained with the molar mass of BaSO4 -233.38g/mol-
Moles Na2SO4:
500cm³ = 0.500L * (0.250mol / L) = 0.125 moles
Moles BaCl2 -Molar mass: 208.23g/mol-
15.00g * (1mol / 208.23g) = 0.072 moles
The moles of BaSO4 are 0.072 moles and its mass is:
0.072 moles * (233.38g / mol) = 16.8g ≈ 16.776g
A chemistry teacher mixed two clear substance together and notices a solid forming at the bottom of the beaker. Is the teacher demonstrating a physical or chemical change?
A. Chemical change because a precipitate was formed
B. Physical change because two substances were mixed together
C. Chemical change because two substances were mixed together
D. Physical change because a precipitate was formed.
Answers
Answer:A. Chemical change because a precipitate was formed.
Explanation:It is a chemical change as we notice that a solid is forming at the bottom of the beaker. Hence, it is a chemical change.
And as we know that chemical change are irreversible change and come out with new products always.
And precipitate are thea solid formed by a change in a solution, often due to a chemical reaction or change in temperature that decreases solubility of a solid.
2. A 20-year-old woman goes to the Emergency Department due to symptoms of palpitations, dizziness, sweating, and paresthesia that have not resolved over the past several days. Her history suggests an anxiety disorder, and blood gases and electrolytes are ordered. Her doctor prescribes a benzodiazepine after a positron emission tomography (PET) scan shows increased perfusion in the anterior end of each temporal lobe. Which of the following blood gases would be expected at the time of admission of this patient?
A. pH 7.51; Pa co: 49 mm Hg: [HCO3] = 38 mEq/L; Anion Gap - 12 mEq/L
B. pH 7.44; Pa co2-25 mm Hg; [HCO3] = 16 mEq/L; Anion Gap = 12 mEq/L
C. pH 7.28: Pa coz 60 mm Hg: [HCO3] =26 mEq/L; Anion Gap = 12 mEq/L
D. pH 7.28: Pa co2 20 mm Hg: [HCO3] = 16 mEq/L: Anion Gap = 25 mEq/L
E. pH 7.51: Pa co2 20 mm Hg: [HCO3] = 24 mEq/L; Anion Gap = 12 mEq/L
Answers
The expected blood gas values for this patient at the time of admission of patient is option E. pH 7.51; PaCO₂ = 20 mm Hg; [HCO₃]⁻ = 24 mEq/L; Anion Gap = 12 mEq/L
A 20-year-old woman presents to the Emergency Department with persistent symptoms of palpitations, dizziness, sweating, and paresthesia. She has a history suggestive of an anxiety disorder.
To assess her condition, blood gases and electrolytes are ordered, and a positron emission tomography (PET) scan is performed. The PET scan reveals increased perfusion in the anterior portion of each temporal lobe. Based on these findings, the doctor prescribes a benzodiazepine medication.
The expected blood gas values at the time of admission can be determined by analyzing the given options:
A. pH 7.51; PaCO₂ = 49 mm Hg; [HCO₃]⁻ = 38 mEq/L; Anion Gap = 12 mEq/L
B. pH 7.44; PaCO₂ = 25 mm Hg; [HCO₃]⁻ = 16 mEq/L; Anion Gap = 12 mEq/L
C. pH 7.28; PaCO₂ = 60 mm Hg; [HCO₃]⁻ = 26 mEq/L; Anion Gap = 12 mEq/L
D. pH 7.28; PaCO₂ = 20 mm Hg; [HCO₃]⁻ = 16 mEq/L; Anion Gap = 25 mEq/L
E. pH 7.51; PaCO₂ = 20 mm Hg; [HCO₃]⁻ = 24 mEq/L; Anion Gap = 12 mEq/L
By evaluating the options, the most appropriate choice is:
E. pH 7.51; PaCO₂ = 20 mm Hg; [HCO₃]⁻ = 24 mEq/L; Anion Gap = 12 mEq/L
This option presents a higher pH (alkalosis) and a decreased PaCO₂ (respiratory alkalosis), which could be consistent with the patient's symptoms of hyperventilation due to anxiety. The [HCO₃]⁻ level within the normal range and a normal anion gap further support this interpretation.
In summary, the expected blood gas values for this patient at the time of admission are a higher pH, decreased PaCO₂, normal [HCO₃]⁻, and a normal anion gap, indicative of respiratory alkalosis likely caused by hyperventilation related to her anxiety disorder.
To know more about Blood gas values here: https://brainly.com/question/27826544
#SPJ11
which stable element is used to determine the age of volcanic rock?
Answers
Answer:One of the most common methods to date volcanic rocks uses potassium (radioactive parent) and argon (stable daughter). Potassium is an element found in many minerals and rocks, and it normally has an atomic mass of 39.
Explanation:
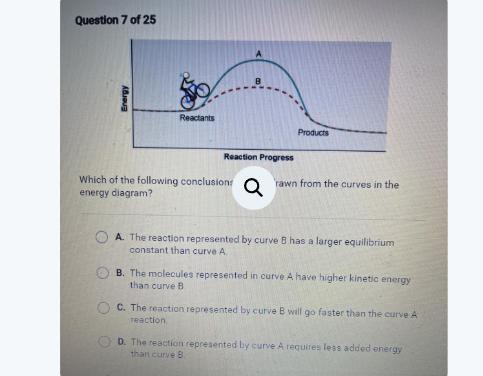
Energy
Reactants
Products
Reaction Progress
Which of the following conclusions can be drawn from the curves in the
energy diagram?
OA. The reaction represented by curve B has a larger equilibrium
constant than curve A.
▸
B. The reaction represented by curve A requires less added energy
than curve B.
OC. The molecules represented in curve A have higher kinetic energy
than curve B.
OD. The reaction represented by curve B will go faster than the curve A
reaction.

Answers
The correct answer is that The reaction represented by curve B will go faster than the curve A reaction.
The energy diagramAn energy diagram illustrates the transfer or utilization of energy. In particular, it depicts the change in energy during a chemical reaction and is sometimes referred to as a reaction progress curve.
In the given energy diagram, there is a representation of a person bicycling. Notably, curve A is positioned higher than curve B. This height difference indicates that bicycling along curve A requires a greater amount of energy compared to bicycling along curve B. Conversely, curve B necessitates a lower amount of energy for the bicycling activity.
Read mroe on energy diagram here https://brainly.com/question/21047184
#SPJ1
You come across two unknown plants and compare their leaves. Plant A has feather like leaves, while Plant B has needles. What type of plant is Plant B? Fern Flowering plant Pine tree Spores
Answers
Answer:
The correct answer is - Pine tree.
Explanation:
Coniferous trees or cone-bearing trees normally called conifers adapted their leaves and have needles like leaves that retain more water. Needles do not appear to be leaves but are modified leaves.
Conifer trees adapt this leaf modification to prevent water loss due to transpiration in the case of dry air or atmosphere. Important members of the conifer trees are pine trees, cedars, spruces, and Pines.
PLEASE HELP I THIS IS DUE TOMORROW AND I LITERALLY DONT KNOW WHAT TO DO FOR PRO AND IGNORE 4 IF YOU CAN CHANGE THAT ANSWER THEN THATS GREAT JUST PLS HELP i don’t think this is chemistry but this is just science ig JUST PLEASE HELP

Answers
Answer: aint no way you really put positive but anywya um on the benefits side, fracking increases economic activity, employment, income and housing prices. But, it also brings more truck traffic, increases in crime and potential health impacts possibly due to air and/or water pollution.
use his but put it in your own words
Explanation: yes
1-I can be a little more specific- we do NOT have enough evidence that fracking, as most induced earthquakes are not directly caused by hydraulic fracturing. Even though fracking can release many harmful gases such as methane, it cannot cause seismic waves
2-Research reveals that fracking increases regulated contaminants found in drinking water, but not enough to trigger regulatory violations. However, fracking can affect our underwater stores, mostly through the harmful gases like methane that can contaminate the water and have negative effects on people who drink it.
3- Because fracking releases a massive amount of harmful gases, the community can experience negative effects such as toxic air and health issues. (You should probably support the con sides as there are little reasons fracking is good)
4-Fracking has negative effects on the atmosphere. In fact, there are very little pros about fracking. It release harmful gases into the air, which can further increase the intoxication the air has.
5-Oil and gas development is primarily regulated under multiple federal environmental and public health laws. These laws apply to drilling and hydraulic fracturing from unconventional sources. However, fracking, despite its limitations, still affects the environment.
WILL GIVE BRAINLIST IF RIGHT
While visiting Thor's Hammer in Bryce Canyon National park, you observe red coloring on the landform. What natural process most likely caused the red coloring? (4 points)
Chemical weathering
Deposition
Erosion
Mechanical weathering
Answers
Answer:
Chemical weathering because the landform changed color
Explanation:
Answer: A) Chemical Weathering
Explanation:
I took the test :)

what is the classification of mixture
Answers
Answer:
classification of mixtures will be: solutions, suspensions, and colloids
Hopefully this helped
What property of a metal does the image represent
Answers
Answer:
malleable
Explanation:
The image represent in malleable property of metal.
The image possibly represents the photoelectric effect of a metal, which is when it emits electrons after being exposed to electromagnetic radiation. Metals are also characterized by physical properties such as conductivity, malleability, metallic luster, and metallic bonding.
Explanation:Based on your question, the image possibly represents the photoelectric effect, a key property of metals. This phenomenon occurs when a metal surface exposed to electromagnetic waves of a certain frequency absorbs radiation and emits electrons. These emitted electrons are called photoelectrons. Metals can also exhibit free electron model behavior, where electrons freely roam within the metal structure.
Metals possess unique physical properties like conductivity, malleability, and metallic luster. Malleability refers to the metal's ability to deform without breaking, while conductivity refers to the metal's ability to transfer heat or electricity. A metallic luster gives metals their characteristic shiny appearance.
Finally, metals are also known for their metallic bonding—a unique force that holds together the atoms within a metallic solid. Metallic bonding gives rise to many useful and varied bulk properties of metals.
Learn more about Properties of Metals here:https://brainly.com/question/33514448
#SPJ2
Which of the following acids will not dissociate completely in water? Pick only one. HCl HClO4 HClO HNO3
Answers
HClO will not dissociate completely in water among the given option.
When acids dissolve in water, they can dissociate into ions. Strong acids dissociate completely, while weak acids only partially dissociate. To determine which acid will not dissociate completely, we need to identify the weak acid among the options.
HClO is a weak acid known as hypochlorous acid. It does not dissociate completely in water. Instead, it partially dissociates into H⁺ and ClO⁻ ions.
On the other hand, HCl, HClO₄, and HNO₃ are strong acids and dissociate completely in water, producing H⁺ ions. These strong acids are considered to be fully ionized in aqueous solutions.
learn more about acids here:
https://brainly.com/question/29796621
#SPJ11
The mass of a book is 8.0 g, the volume is 4.0 cm^3 What is the density of the book?
Use the formula D=M/V
Answers
Answer:
2 g/mL
Explanation:
d = m/v
d= 8/4
d=2
hope that helps!
Multi-part question for my lab that I just can't figure out. Can you please give me an explanation to all steps within this problem.
A. Calculate the thickness of the monolayer assuming that the volume of the monolayer is 7.43×10−6 mL and the diameter of the watch glass is 5 cm.B.
B. Determine the number of moles of oleic acid in the monolayer. Assume the number of grams of oleic acid in the monolayer is 7.52×10−6 g .
C. Calculate the surface area of one molecule if we assume the molecule is shaped like a cylinder that the hight is 10x larger than the radius.
D. Determine the area of the surface covered by the molecules assuming they have a circular surface which at most can cover 90.6%.
Answers
A Radius = 0.025 m, B Number of moles = 2.662×10−8 mol, C r = √(Surface Area / (22π)), D Area covered = 0.001778863 \(m^2\) . To calculate the thickness of the monolayer, we need to know the volume of the monolayer and the surface area it covers.
We are given the volume of the monolayer, which is 7.43×10−6 mL . The surface area covered by the monolayer can be calculated by dividing the volume by the diameter of the watch glass, which is 5 cm or 0.05 m.
Surface area = Volume/Diameter = (7.43×10−6 mL) / (0.05 m) = 1.486×10−7 m²
The thickness of the monolayer can then be calculated by dividing the volume of the monolayer by the surface area it covers.
Thickness of monolayer = Volume / Surface area = (7.43×10−6 mL) / (1.486×10−7 m²) = 0.05 mm
B. To determine the number of moles of oleic acid in the monolayer, we are given the number of grams of oleic acid in the monolayer, which is 7.52×10−6 g. The molar mass of oleic acid is 282.46 g/mol. We can use these values to calculate the number of moles of oleic acid in the monolayer.
Number of moles = Mass / Molar mass = (7.52×10−6 g) / (282.46 g/mol) = 2.66×10−8 mol
C. To calculate the surface area of one molecule of oleic acid, we are given that the molecule is shaped like a cylinder with a height 10 times larger than the radius. We can assume that the length of the oleic acid molecule is the height of the cylinder and the diameter of the oleic acid molecule is the diameter of the cylinder.
Let the radius of the cylinder be 'r'. Then, the height of the cylinder is 20r.
The surface area of the cylinder can be calculated as follows:
Surface area = 2πr² + 2πr(20r) = 2πr(41r) = 82πr²
D. To determine the area of the surface covered by the molecules, we need to use the information that the circular surface can cover at most 90.6% of the total surface area. Let A be the total surface area covered by the molecules, and let x be the surface area not covered by the molecules. Then, we can write:
A + x = total surface area
We know that x is 9.4% of the total surface area, so we can write:
x = 0.094 * total surface area
Substituting this into the first equation, we get:
A + 0.094 * total surface area = total surface area
Simplifying, we get:
A = 0.906 * total surface area.
To learn more about monolayer refer to this link
https://brainly.com/question/14699941
#SPJ4
A sample of gas of mass 2.929g occupies a volume of 426mL at 0°C and 1.00atm pressure.what is molecular weight of the gas?
Answers
Answer:
154 g/mole
Explanation:
We are given the mass of the gas, but we also need the number of moles the 2.929g represents. Since we are provided the conditions of the gas, we can the Ideal Gas law to find the number of moles of the mystery gas.
Ideal Gas Law: PV = nRT, where P, V, and T are the pressure, volume, and temperature (temperature must be in degrees Kelvin), n is the moles, and R is the gas constant.
Let's choose the gas constant that has the same units as we were given. R = 0.0820575 [L⋅atm⋅/(K⋅mol)] comes the closest, but we'll still need to convert ml to liters(L) and °C to °K:
426mL = 0.426L
0°C = 273.25 [add 273.15 to the Centrigrade value]
Let's rearrnage the ideal gas law to solve for n, the number of moles:
n = (PV/RT)
Now enter the data:
n = (1atm)(0.426L)/[(0.0820575 L⋅atm⋅/(K⋅mol))*(273.15°K)]
n = (1atm)(0.426L)/[(0.0820575 L⋅atm⋅/(K⋅mol))*(273.15°K)] [Units that cancle are highlighted]
n = (1)(0.426)/[(0.0820575 /(mol))*(273.15)] [We are left only with moles (mol)
n = (0.426)/(0.0820575)/(273.15) [1/1/mol] [Move the only unit out (1/1/mol)]
n = (0.426)/(0.0820575)/(273.15) [1/1/mol] = 0.0190 moles
Note that the unit moves to the top, i.e., : 1/1/mol = mole
We have the mass and the number of moles. Divide the two to obtain molar mass:
(2.929g)/(0.0190 moles) = 154 g/mole This is also the molecular weight.
[I don't know what is a gas at 0°C and has that molecular weight]
What does it mean for a strong base to be in equilibrium?
o the position of equilibrium lies far to the right, with products being favored.
o the position of equilibrium lies far to the left, with products being favored.
there is a great deal of base and very few ions.
o there is an equal amount of reactants and products.
mark this and retum
save and exit
next
submit
Answers
The position of equilibrium lies far to the right, with products being favoured. Hence, option A is correct.
What is equilibrium?Chemical equilibrium is a condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and products occurs.
A very high value of K indicates that at equilibrium most of the reactants are converted into products.
The equilibrium constant K is the ratio of the concentrations of products to the concentrations of reactants raised to appropriate stoichiometric coefficients.
When the value of the equilibrium constant is very high, the concentration of products is much higher than the concentration of reactants.
This means that most of the reactants are converted into products and the position of equilibrium lies far to the right, with products being favoured.
Hence, option A is correct.
Learn more about the equilibrium here:
https://brainly.com/question/23641529
#SPJ1
the ionization energy of o2 is 1205 kj/mol. what is the maximum wavelength of light capale of causing the ionization of o2?
Answers
O2 can only be ionized by light with a maximum wavelength of 99.39 nm.
What is ionization energy?The ionization energy measures an element's ability to participate in chemical processes that call for the creation of ions or the donation of electrons.
ionization energy of O2 = 1205 kJ
energy = hv
= h × wavelength/speed of light
wavelength = 1205000× 3 ×10⁸ /6.626 ×10³⁴
wavelength = 99.39 nm.
He is symbolized by the highest ionization energy. The outermost shell has a high ionization energy, is stable, and does not frequently become unstable due to electron loss. Ionization can be induced by waves with energies greater than 134 nm. 225nm light lacks the energy to ionize gold because it is greater than 134nm.
To know more about ionization energy, visit:
https://brainly.com/question/28385102
#SPJ4
What volume of o2 is produced when 28.5 g of hydrogen peroxide decomposes to form water and oxygen at 150 degrees c and 2.0 atm?
Answers
Taking into account the reaction stoichiometry, the volume of O₂ is 7.28406 L when 28.5 g of hydrogen peroxide decomposes to form water and oxygen at 150 degrees c and 2.0 atm.
Reaction stoichiometryThe balanced reaction is:
2 H₂O₂ → 2 H₂O + O₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
H₂O₂: 2 molesH₂O: 2 molesO₂: 1 moleThe molar mass of the compounds is:
H₂O₂: 34 g/moleH₂O: 18 g/moleO₂: 32 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
H₂O₂: 2 mole× 34 g/mole= 68 gramsH₂O: 2 moles× 18 g/mole= 36 gramsO₂: 1 mole× 32 g/mole= 32 gramsMass of O₂ formedThe following rule of three can be applied: if by reaction stoichiometry 68 grams of H₂O₂ form 1 mole of O₂, 28.5 grams of H₂O₂ form how many moles of O₂?
moles of O₂= (28.5 grams of H₂O₂×1 mole of O₂)÷68 grams of H₂O₂
moles of O₂= 0.42 grams
Then, 0.42 moles of O₂ are formed.
Definition of ideal gas lawIdeal gases are a simplification of real gases that is done to study them more easily. It is considered to be formed by point particles, do not interact with each other and move randomly. It is also considered that the molecules of an ideal gas, in themselves, do not occupy any volume.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P×V = n×R×T
Where:
P is the gas pressure.V is the volume that occupies.T is its temperature.R is the ideal gas constant. The universal constant of ideal gases R has the same value for all gaseous substances. n is the number of moles of the gas. Volume of O₂In this case, you know:
P= 2 atmV = ?n= 0.42 molesR= 0.082 (atmL)÷(molK)T= 150 C= 423 KReplacing in the definition of the ideal gas law:
2 atm×V = 0.42 moles×0.082 (atmL)÷(molK)× 423 K
Solving:
V = (0.42 moles×0.082 (atmL)÷(molK)× 423 K)÷ 2 atm
V= 7.28406 L
Finally, the volume of O₂ is 7.28406 L.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ4
CHEM ASSIGNMENT!!
PLEASE HELP

Answers
Answer:
Explanation:
q=mcat
q= 50g(4.184)(100-100)
q = 0
What is the molarity of p-nitroaniline in a solution if the absorbance is 0.233 assuming a path length of 1.00 cm ? The molar absorptivity for p-nitroaniline can be found immediately before the experimental section in Experiment B. Include units in answer. Use 3 significant figures for answer.
Answers
The molarity of p-nitroaniline in a solution if the absorbance is 0.233 assuming a path length of 1.00 cm is 0.0809 M.
How to find molarity of p-nitroaniline in a solution?
The molarity is the measure of the number of moles of solute per unit volume of a solution.
The formula for molarity is:
Molarity = Moles of solute / Volume of solution (in liters)
Given data:
Absorbance = 0.233
Path length = 1.00 cm
Molar Absorptivity = 7,700 L / mol * cm (given in the experimental section)
Molar mass of p-nitroaniline = 139.11 g/mol
To calculate the molarity of p-nitroaniline in a solution, we have to first calculate the concentration of p-nitroaniline (in mol/L).
To calculate the concentration of p-nitroaniline, we have to use the Beer-Lambert law.
A = εlcwhere, A = absorbance, ε = molar absorptivity, l = path length, and c = concentration Rearranging the formula:
c = A / εlc = 0.233 / (7,700 L/mol*cm × 1.00 cm)c
= 0.0000302650 mol/L Molarity
= Moles of solute / Volume of solution (in liters)Moles of p-nitroaniline
= (Concentration × Volume of solution) / Molar mass of p-nitroaniline Moles of p-nitroaniline
= (0.0000302650 mol/L × 1000 mL) / 139.11 g/mol Moles of p-nitroaniline
= 0.00021884 mol/L
= 2.1884 × 10-4 L Molarity
= Moles of solute / Volume of solution (in liters)Molarity
= 2.1884 × 10-4 L / 1.00 L Molarity
= 0.0809 M
Thus, the molarity of p-nitroaniline in a solution if the absorbance is 0.233 assuming a path length of 1.00 cm is 0.0809 M.
#SPJ11
Learn more about p-nitroaniline:
https://brainly.com/question/17114364
Which of the following are functions of the stomach?
Answers
Answer:
1-Temporarily store food.
2-Contract and relax to mix and break down food.
3-Produce enzymes and other specialized cells to digest food
anyone know this? i’m having trouble...please help.

Answers
Why because the last two are wrong there is no way they are common when they have different genes the first one is wrong too so the second
nitrogen gas escapes through a pinhole in 83.8 seconds. under the same conditions, a gaseous compound with the empirical formula ch2 escapes in 68.4 seconds. what is its molecular formula?
Answers
The molecular formula of the compound is \(C_2H_3\).
To determine the molecular formula of the gaseous compound, we need to use the information given about its empirical formula and the time it takes to escape through a pinhole and apply the concept of the Ideal Gas Law.
The empirical formula of the compound tells us the simplest whole-number ratio of the atoms present in the molecule. In this case, the empirical formula is \(CH_2\), which means that the molecule contains one carbon atom and two hydrogen atoms.
The time it takes for gas to escape through a pinhole is related to its molar mass and the size of the hole. The lighter the gas, the faster it will escape. Therefore, we can compare the escape times of nitrogen gas and the compound to determine their relative molar masses.
Using the Ideal Gas Law, we can relate the molar mass of the compound to the time it takes to escape through the pinhole. Assuming the same conditions of temperature and pressure, the Ideal Gas Law states that PV=nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature. Rearranging this equation gives us n=m/M, where m is the mass of the gas and M is the molar mass.
We can use this equation to find the molar mass of the compound:
n = m/M
n = m/ (Empirical formula mass of \(CH_2\))
n = m/ (12.01 + 2*1.01)
n = m/ 14.03
We know that the time it takes for nitrogen gas to escape through the pinhole is 83.8 seconds, and the time for the compound is 68.4 seconds. Therefore, the ratio of their molar masses is:
(Molar mass of nitrogen gas) / (Molar mass of the compound) = (Time for the compound to escape) / (Time for nitrogen gas to escape)
(Molar mass of nitrogen gas) / (Molar mass of the compound) = 83.8 / 68.4
(Molar mass of nitrogen gas) / (Molar mass of the compound) = 1.2246
We know the molar mass of nitrogen gas is 28.01 g/mol, so we can solve for the molar mass of the compound:
28.01 / (Molar mass of the compound) = 1.2246
The molar mass of the compound = 22.87 g/mol
Now we can use the molar mass of the compound to find its molecular formula. The empirical formula mass of \(CH_2\) is 14.03 g/mol, so the molecular formula mass must be a multiple of this value that is close to 22.87 g/mol. Dividing 22.87 by 14.03 gives a value of 1.63, which suggests that the molecular formula contains approximately 1.63 times as many atoms as the empirical formula.
To find the molecular formula, we can multiply the empirical formula by this factor:
(\(CH_2\)) x 1.63 = C1.63H3.26
Rounding to the nearest whole number, we get the molecular formula \(C_2H_3\).
To learn more about molecular
https://brainly.com/question/30640129
#SPJ4
What are the major disadvantages of using ozone instead of chlorine to disinfect water? O Ozonation is more expensive than chlorination and ozone leaves an odor in the water O Ozonation causes trihalomethane formation and leaves an odor in the treated water O Ozone decomposes quickly and does not provide long-term protection against possible contamination as the water is piped through a municipal distribution system O Ozonation causes trihalomethane formation and is more expensive than chlorination
Answers
The major disadvantage is - Ozone decomposes quickly and does not provide long-term protection against possible contamination as the water is piped through a municipal distribution system.
The gas ozone (O3) is unstable. As a result, it breaks down quickly, oxidizing any organic impurities present in the water including bacteria, viruses, and germs, but because it is a gas, it leaves the water after the oxidation is complete. Therefore, when the ozone started to disintegrate at first, the water may have various additional contaminants that mix with it as it travels through the municipal distribution system. Water carries through the new pollutants from the pipes. Ozone therefore has no long-term effect.
Chlorination produces trihalomethane, not ozonation, hence the expansiveness of ozone is dependent on the impurity of the water. Trihalomethane is produced by chlorination, not ozonation. Although ozone as a gas has a faint odor, when it is combined with water, it oxidizes the pollutant and breaks down, leaving no ozone in the water. Therefore, water does not smell like ozone.
Learn more about ozonation:
brainly.com/question/27911475
#SPJ4