Answers
We have to look for safe and non - hazardous characterstics when trying to find new propellants.
What are propellants ?Propellants are any gas, liquid, or solid the expansion of which can be used to impart motion to another substance or object.
The Desirable properties for propellant combinations are ;
low molecular mass and high temperature of reaction products (for high exhaust velocity), high density (to minimize tank weight), low hazard factor (e.g., corrosivity and toxicity), low environmental impact, low cost.Learn more about propellants here ;
https://brainly.com/question/1395855
#SPJ1
Related Questions
what cases might humans need to collaborate with computers to solve a problem
Answers
Answer:
Like when they work of need to look something important
Explanation:
like image you are chilling and then your like omg I need to turn in the work and you go to your computer and send it but you used your computer
Ok help it’s for homework

Answers
Explain how a rainbow is produced
Answers
A rainbow is produced through a proces that includes refraction, reflection, and dispersion of sunlight.
What more should you know about the production of rainbows?A rainbow is formed when sulinght is refracted and reflected by rain drops in the atmospher.
The sunlight is split into its component colors, which is why rainbows appear as having an array of colors. This is due to each color being bent by a different amount during refraction.
The colors of a rainbow are always in the same order, with red on the outside and violet on the inside.
Find more exercises on rainbows;
https://brainly.com/question/7965811
#SPJ1
_______ capacity is a term used to describe the ability of a solution to prevent large changes in pH with the addition of a base or acid.
a) Heat
b) Buffering
c) Vaporization
d) Cohesive
e) Freezing
Answers
Buffering capacity measures a solution's capability to withstand pH fluctuations by either absorbing as well as desorbing H+ as well as OH- ions.
Aqueous buffer solutions were made up of a weak acid as well as its conjugate base or even a weak base as well as its conjugate acid. The capability of buffer solutions to keep a fairly constant pH value in reaction to the addition of a tiny amount of acid or base is an important attribute.
A buffer would be a solution that avoids pH changes when a minuscule portion of strong acid as well as strong base is added. Technical definition (How does one come up with one?): A buffer was made up of a weak acid and its conjugate base.
Thus, the correct answer will be option (b).
To know more about solution's
https://brainly.com/question/1616939
#SPJ4
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
KCIO3 -> KCI + 02
How many moles of KCI are produced if 6743 grams of KCIO3 decomposes?
Answers
55.03 moles of KCI are produced when 6743 grams of \(KClO_{3}\) decomposes
To determine the number of moles of KCl produced when 6743 grams of \(KClO_{3}\) decomposes, we need to use the concept of molar mass and the balanced chemical equation.
First, let's calculate the molar mass of \(KClO_{3}\)
The molar mass of potassium (K) is approximately 39.10 g/mol.
The molar mass of chlorine (Cl) is approximately 35.45 g/mol.
The molar mass of oxygen (O) is approximately 16.00 g/mol.
So, the molar mass of \(KClO_{3}\) is:
(39.10 g/mol) + (35.45 g/mol) + (3 * 16.00 g/mol) = 122.55 g/mol.
Now, we need to calculate the number of moles of \(KClO_{3}\):
Number of moles = Mass / Molar mass
Number of moles = 6743 g / 122.55 g/mol = 55.03 mol.
According to the balanced chemical equation:
2\(KClO_{3}\) -> 2 KCl + 3 O2,
we can see that for every 2 moles of \(KClO_{3}\), we obtain 2 moles of KCl.
Therefore, the number of moles of KCl produced will be equal to the number of moles of \(KClO_{3}\) since the ratio is 1:1. Thus, 55.03 moles of KCl will be produced.
Know more about molar mass here:
https://brainly.com/question/837939
#SPJ11
explain why it is important not to correct any gas from the first few seconds of the experiment
Answers
Answer:
gu kha fuschhehdjdvdbeodbr
You notice that every time you place butter on your stack of freshly made pancakes, the butter melts.
According to the law of conservation of energy, heat lost by the pancake
Answers
Answer:
Heat lost by the pancake = Heat gained by butter
Explanation:
Because energy is always conserved, this process involves the loss of energy by one substance which is gained by another.
The pancakes were freshly made, so they are hot; having the required energy to melt the butter placed on them. The butter absorbs heat from the pancakes which is equal to or greater than its melting point. Thus, the butter melts due to the heat absorbed.
Therefore,
Heat lost by the pancakes = Heat gained by butter
HELPPP
Engineering includes the study of:
science, math, universe, and history
science, universe, space, and logic
science, math, logic, and economics
science, math, history, and space
Answers
Engineering includes the study of science, universe, space, and logic and is therefore denoted as option B.
What is Engineering?This is referred to as the branch of science and technology concerned with the design, building, and use of engines, machines, and structures which helps to make work easier and faster.
It is also studied in schools and has various divisions such as mechanical, chemical engineering etc and it involves the study of science, universe, space, and logic which are encountered when dealing with this broad subject and is therefore the reason why option B was chosen as the most correct choice.
Read more about Engineering here https://brainly.com/question/17169621
#SPJ1
Adaleigh is learning about renewable and non-renewable resources in her science class. She decides to take
a nature walk and makes notes of all the renewable resources that she sees. Which of the following is an
observation that Adaleigh could have made on her walk?
A. The seeds on the ground are going to grow into new trees.
B. The tallest trees had the most seeds around their bases.
C. When trees decay, they could eventually turn into coal.
D. Pine trees are older than oak trees.
Answers
Answer:
A. The seeds on the ground are going to grow into new trees.
Explanation:
Trees are renewable resources.
Who is the Scientist that came up with the idea of natural selection?
Answers
sodium chloride is made from sodium and chloride. would you expect the properties of sodium chloride to be simliar to sodium or cloride
Answers
Answer:
No
Its a chemical change so the characteristics or chemical properties always differs thereby the properties of sodium chloride isn't similar to sodium or chloride
as heat is added, the pressure in this gas . view available hint(s)for part a as heat is added, the pressure in this gas . increases decreases remains constant cannot be determined
Answers
The ideal gas law can be reorganized to arrive at: assuming that the volume and molecular weight of the gas remain constant:
P/T = constant indicates that the gas's pressure must rise with its temperature in order to maintain a constant ratio of pressure to temperature.
Consequently, if we heat the gas while maintaining its volume and molecular weight, its pressure will rise.
How does temperature work?In other words, it is a measure of how quickly the particles of a substance are moving. Temperature is a fundamental physics concept that can be measured in Kelvin (K), Fahrenheit (°F), and Celsius (°C) scales, among others.
On the Celsius scale, water boils at 100°C and freezes at 0°C at normal atmospheric pressure. On the Fahrenheit scale, water boils at 212°F and freezes at 32°F at normal atmospheric pressure. On the Kelvin scale, 0 K is the theoretical minimum temperature at which all particles have no kinetic energy.
To know more about Temperature:
brainly.com/question/23411503
#SPJ1
Stamples of heterogeneous equilibria. FeO(s) + CO(g) = Fe(s) + CO₂(g) II. H₂(g) L₂(g) = 2HI(g) III. CO₂(g) + C(s) = 2CO(g) IV. N₂(g) 3H₂(g) + 2NH3(g) Identify I.
Answers
An example of heterogeneous equilibrium is:
I. FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g)What is heterogeneous equilibrium?Heterogeneous equilibrium refers to an equilibrium state in a chemical reaction where the reactants and products exist in different physical states or phases. It occurs when substances in different phases, such as solids, liquids, and gases, are involved in a chemical reaction.
Considering the given equations:
The equation I: FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g) represents a heterogeneous equilibrium.
This is because the reactants and products involve different phases (solid and gas). FeO is a solid (s), CO is a gas (g), Fe is a solid (s), and CO₂ is a gas (g). The reaction involves the conversion of a solid and a gas to another solid and a gas, and the equilibrium is established between these different phases.
Learn more about heterogenous equilibrium at: https://brainly.com/question/25257772
#SPJ1
H2SO, is an example of which of the following?
A) Element
B) Compound
C)Noble Gas
D)Mixture
Answers
Answer:
The answer is B.
Explanation:
Its a chemical compound
Which of the steps in prokaryotic binary fission is correct?
Question 11 options:
a) All of these choices are correct.
b) The two replicated chromosomes remain attached to the plasma membrane.
c) The cell continues to grow outward symmetrically, separating the two chromosomes.
d) Cell wall material is laid down at the midpoint to separate the two daughter cells.
e) DNA is replicated bidirectionally from a single point on the circular chromosome.
Answers
From a single location on the circular chromosome, DNA is copied in both directions.
The correct option is E.
What is of binary fission?Binary fission is the process of asexual reproduction in which one organism is divided into two separate ones. An organism's genetic material, or deoxyribonucleic acid (DNA), doubles when it splits into two halves (cytokinesis) through binary fission, with each new species inheriting one copy of the latter.
What cells use binary fission?Bacterial binary fission is the method by which bacteria split their cells. Find out how binary fission functions, including how to make a new cell wall and a copy of a bacterial chromosome. Mitosis and binary fission, two types of asexual reproduction, both entail the division of a parent cell into two identical daughter cells.
To know more about Binary fission visit:
https://brainly.com/question/7639952
#SPJ1
The Sun has been shining on this swimming pool all day. The water is much warmer than it was in the morning. Describe what is happening to the water in terms of temperature, particle speed, and kinetic energy.
Answers
Answer:
The waters' temp increased
Explanation:
The temperature of the water in the swimming pool has increased due to the heat from the Sun. As a result, the particles in the water are moving faster and have a higher kinetic energy than in the morning.
The density of an object is 5g/cm^3 and the volume of the object is 10 cm^3. What is the mass of the object
Answers
The mass of the object of density 5g/cm³ and volume 10 cm³ is 50kg.
What does physics mean when it refers to density?Defining density How tightly a material is packed together is determined by its density. Its definition is "mass per unit volume". D or, the symbol for density
Density Formula: = m/V,
where is the density, m is the object's mass, and V is its volume.
The units used change depending on the calculation's use of mass and volume units. Density would be expressed in kg/cm³ if the mass is expressed in kg and the volume in cm³.
Density = mass / volume
Mass = density × volume
Mass = 5×10
Mass = 50 kg.
To know more about density, visit:
https://brainly.com/question/6107689
#SPJ1
I WILL GIVE 30 POINTS TO THOSE WHO ANSWER THIS QUESTION RIGHT NOOOO SCAMS PLEASE

Answers
The general gas equation, commonly referred to as the ideal gas law, represents the state of a fictitious ideal gas through an equation. Here the mass of helium gas required to pressurize 86 L tank to 201 atm is 2561.8 g.
According to the ideal gas law, the sum of the absolute temperature of the gas and the universal gas constant is equal to the product of the pressure and volume of one gram of an ideal gas.
The ideal gas equation is given as:
PV = nRT
n = PV / RT
56°C = 329 K
n = 201 × 86 / 0.08206 × 329 = 640.45 mol
Molar mass of 'He' = 4.00 g / mol
Mass = 640.45 × 4.00 = 2561.8 g
To know more about ideal gas law, visit;
https://brainly.com/question/30458409
#SPJ1
What is the change in temperature (AT) when a 25 g block of aluminum absorbs 10,000 J of heat?
Answers
The change in temperature (ΔT) when a 25 g block of aluminum absorbs 10,000 J of heat is approximately 44.32°C.
To calculate the change in temperature (T) that occurs when an aluminium block absorbs a certain quantity of heat, we must utilise the specific heat capacity of aluminium (c) and the equation:
Q = mcΔT
Where Q is the heat absorbed or released, m is the substance's mass, c is the substance's specific heat capacity, and T is the temperature change.
The specific heat capacity of aluminium is approximately 0.897 J/g°C.
Given that the aluminium block weighs 25 g and absorbs 10,000 J of heat, we can plug the following values into the equation:
(25 g) * (0.897 J/g°C) * T = 10,000 J
We can now solve for T:
T = 10,000 joules / [(25 g) * (0.897 J/g°C)]
ΔT ≈ 44.32°C
for more questions on temperature
https://brainly.com/question/4735135
#SPJ11
What is the mole ratio of hydrogen to nitrogen in the following reaction: N2(g) + 3H2(g) →
2NH3(8)
Answers
Answer:
3 mol H2/2 mol N2
Explanation:
Mole ratios are basically from the numbers/coefficients in front of the element

Arenediazoniums can undergo electrophilic aromatic substitutions with a wide variety of activated aromatic compounds to yield new azo dyes.
a. True
b. False
Answers
I did this last year and it’s false
Take a look at your rubbing alcohol at home. What is its main composition?
Answers
Answer:
It is a mixture of denatured alcohol, water, and agents added to make the alcohol unpalatable to drink.
What volume of 0.180 M KOH is needed to react completely with 15.1 mL of 0.190 M H2SO4
Answers
The volume of 0.180 M KOH that would be needed to completely react with 15.1 mL of 0.190 M \(H_2SO_4\) will be 31.88 mL
Stoichiometric problemThe equation of the reaction involving KOH and \(H_2SO_4\) is as follows:
\(2KOH + H_2SO_4 --- > K_2SO_4 + 2H_2O\)
The equation is already balanced and the mole ratio of KOH and \(H_2SO_4\) is 2:1.
But 15.1 mL of 0.190 M \(H_2SO_4\) is available.
Recall that:
mole = molarity x volume
Mole of 15.1 mL of 0.190 M \(H_2SO_4\) = 0.190 x 15.1/1000
= 0.002869 mol
From the 2:1 mole ratio, the equivalent mol of KOH would then be:
0.002869 x 2 = 0.005738 mol
Since, mole = molarity x volume. Then, volume = mole/molarity
So, the volume of 0.005738 mol of 0.180 M KOH would be:
volume = 0.005738/0.180
= 0.03188 liters
Converting 0.03188 liters to mL:
0.03188 x 1000 = 31.88 mL
Thus, the volume of 0.180 M KOH needed to react completely with 15.1 mL of 0.190 M H2SO4 would be 31.88 mL.
More on stoichiometric problems can be found here: https://brainly.com/question/14465605
#SPJ1
Please help me with this on the picture

Answers
Answer:
Umm … can you make it horizontal Please
Explanation:
cl-+peg=hcl+peg rate law, rate constant k
Answers
a. The rate law for this reaction is: Rate = k[Cl] [H₂]. This means that the rate of the reaction is directly proportional to the concentrations of both Cl and H₂ molecules.
What is rate law?Rate law is an equation that describes the rate of a chemical reaction as a function of the concentrations of reactants. The rate law allows us to describe how the rate of a reaction changes when the concentrations of reactants are changed. It is derived from the rate equation, which is a mathematical expression that can be used to calculate the rate of a reaction from the concentrations of the reactants and the rate constant.
b. The rate law for this reaction is: Rate = k[O] [Os]. This means that the rate of the reaction is directly proportional to the concentrations of both O and Os molecules.
c. The rate law for this reaction is: Rate = k[NO₂]₂. This means that the rate of the reaction is directly proportional to the square of the concentration of NO₂ molecules.
To learn more about rate law
https://brainly.com/question/16981791
#SPJ1
Complete Question:

Q3: Match the column A with Column B and write answer in Column C
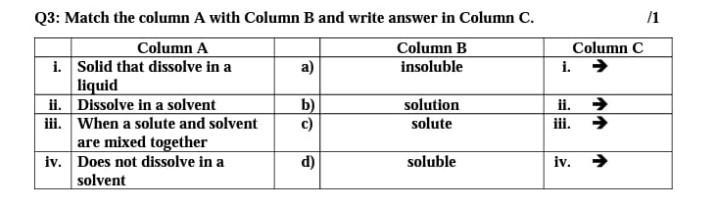
Answers
Answer:
ok
Explanation:
1d
2c
3b
4a
98.96g/mol of CH2O what will be the chemical formula
Answers
Let's break down the molar mass of CH2O:
- Carbon (C) has a molar mass of approximately 12.01 g/mol.
- Hydrogen (H) has a molar mass of approximately 1.01 g/mol.
- Oxygen (O) has a molar mass of approximately 16.00 g/mol.
Now, let's calculate the molar mass of CH2O:
(1 x molar mass of C) + (2 x molar mass of H) + (1 x molar mass of O)
= (1 x 12.01 g/mol) + (2 x 1.01 g/mol) + (1 x 16.00 g/mol)
= 12.01 g/mol + 2.02 g/mol + 16.00 g/mol
= 30.03 g/mol
The molar mass of CH2O is approximately 30.03 g/mol, which is different from the given molar mass of 98.96 g/mol.
It seems that there might be an error or misunderstanding in the given molar mass value. The correct chemical formula for a compound with a molar mass of 98.96 g/mol cannot be determined based on the information provided.
8. What is the density of a rock with a volume of 5 cubic centimeters and a mass of 3 grams?
Answers
Answer:
The answer is 0.6 g/cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\ \)
From the question
mass = 3 g
volume = 5 cm³
We have
\(density = \frac{3}{5} \\ \)
We have the final answer as
0.6 g/cm³Hope this helps you
FeO+ PdF2 ---> FeF2+PdO balance please worth 50 points
Answers
Answer: FeO
Explanation:
Answer:
This is not worth 50 points liar
Explanation: