how many mls of concentrated (18m) sulfuric acid are needed to prepare 1.00 l of 1.00 m sulfuric acid?
Answers
56 ml of concentrated (18 M) sulfuric acid is needed to prepare 1.00 L of 1.00 M sulfuric acid solution.
Initial volume of H₂SO₄ solution (V1) = ?
Initial molarity of H₂SO₄ solution (M1) = 18 M
Final molarity of H₂SO₄ solution (M2) = 1.00
Final volume of H₂SO₄ solution (V2) = 1 L or 1000 ml
To find out the final volume (V2) we will use the following equation
M1V1 = M2V2
Rearrange the equation for initial volume (V1)
V2 = M2V2 / M1
Put the values in the above equation
V1 = 1.0 M × 1000 ml / 18 M
V1 = 1000 / 18 ml
V1 = 56 ml
You can also learn about molarity from the following question:
https://brainly.com/question/16727614
#SPJ4
Related Questions
John needs 6 m of wood to build a diving board. The wood comes in different size packages. Select all the packages John could buy that would meet his needs?
3 pieces at 2000 mm each
4 pieces at 1.5 cm each
6 pieces at 100 mm each
6 pieces at 100 cm each
8 pieces at 200 mm each
Answers
On calculating using conversions, John can select either the package of wood with (a) 3 pieces of 2000mm each or the one with (d) 6 pieces of 100cm each.
On conversion of units from mm and cm to m:
1m = 100cm
1m = 1000mm
Therefore,
Since John requires 6m of wood to build a diving board, using unit conversions:
3 pieces of 2000mm each:
2000mm = 2m = 2m x 3 = 6m
4 pieces of 1.5 cm each:
1.5cm = 0.015m x 4 = 0.06m
6 pieces of 100 mm each:
100mm = 0.1m x 6 = 0.6m
6 pieces of 100 cm each:
100cm = 1m x 6 = 6m
8 pieces of 200 mm each:
200mm = 0.2m x 8 = 1.6m
Result:
The package with 3 pieces wood of 2000mm each equals to 6m of wood.
Similarly, the package with 6 pieces wood of 100cm each equals to 6m of wood.
Learn more about Conversions here:
https://brainly.com/question/12817301
#SPJ9
Explain the process of how James Chadwick
found the neutron.
Answers
Answer:
In 1932, the physicist James Chadwick conducted an experiment in which he bombarded Beryllium with alpha particles from the natural radioactive decay of Polonium. The resulting radiation showed high penetration through a lead shield, which could not be explained via the particles known at that time.
Explanation:
a reaction that produces heat is called and the sign of the enthalpy change is .
Answers
Exothermic Reaction produces heat. The change in enthalpy, ΔH, for an exothermic reaction will always be negative.
Because the total energy of the products is lower than the total energy of the reactants, an exothermic reaction releases energy. A reaction for which the overall standard Gibbs energy change ΔG⚬ is negative. A chemical system's energy is essentially represented by its enthalpy. The heat q that is moved out of (or into) a closed system at constant pressure without any electrical energy input or output is equivalent to the enthalpy change (H) for that reaction. Using calorimetry, such as with a bomb calorimeter, one may determine how much heat is produced or absorbed during a chemical reaction. Reaction calorimeters are frequently used laboratory instruments for measuring heat transfer into or out of reaction vessels. A combustion reaction's heat release and associated energy change, H, can be measured very precisely.
To know more about Enthalpy click here:
brainly.com/question/12676996
#SPJ4
what is the ratio of 32 to 16?
Answers
The ratio of 32 to 16 is:
32:16=> 32/16=> 2/1=> 2:1Conclusion:Hence, 2:1 will be the ratio.
Hoped this helped.
\(BrainiacUser1357\)
What is the molarity of a solution if there are 160.0 g of h2so4 in a 0.500 l solution?
Answers
The molarity of the solution is 0.32 M
Calculation,
Molarity of the solution is the total number of moles present in volume in liter.
molarity = number of moles/volume in liter = n/V ...(i)
Number of moles of sulfuric acid = given mass/molar mass
Number of moles of sulfuric acid (n) = 16g/98.079g/mol = 0.16 mole
Volume of the solution (V) = 0.5 L
Putting the value of volume and number of moles in equation (i) we get,
molarity = 0.16 mol/0.5 L = 0.32 mol/L = 0.32 M
Learn more about molarity
https://brainly.com/question/8732513
#SPJ4
Mark as brainliest if you answer it right.
Samantha noticed her hands get colder in winter than other exposed parts of her body such as her face and neck.

Answers
The element in group 4 and period 3
Answers
help meeeee brainliest for whoever helps

Answers
Answer:
no
Explanation:
Answer:
All A's
Explanation:
it might sound sus but it all a's
Draw the Lewis structure for CO32- including any valid resonance structures. Which of the following statements is TRUE? A) The CO32- ion contains one C-O single bond and two C=O double bonds. B) The CO32- ion contains two C-O single bonds and one C=O double bond. C) The CO32- ion contains three C-O double bonds. D) The CO32- ion contains two C-O single bonds and one C=0 triple bond. E) None of the above are true. 14) How many of the following elements can form compounds with an expanded octet? Pb Kr Si B A) 0 B) 1 C) 2 D) 3 E) 4
Answers
The Lewis structure for CO32- has one carbon atom bonded to three oxygen atoms, with a negative charge on the ion. One of the oxygen atoms is singly bonded to the carbon atom, while the other two oxygen atoms are double bonded to the carbon atom. There are two valid resonance structures for CO32-, where the double bonds are shifted between the different oxygen atoms.
Therefore, the answer is A) The CO32- ion contains one C-O single bond and two C=O double bonds.
Elements in the third period or higher, such as sulfur, phosphorus, and chlorine, can form compounds with an expanded octet. However, elements in the second period, such as boron and carbon, typically do not form compounds with an expanded octet.
Therefore, the answer is B) 1 (only the element Pb can form compounds with an expanded octet).
Learn more about oxygen atoms:
https://brainly.com/question/28009615
#SPJ11
Corrosive chemicals usually involve what kind of reaction(s)?
- Acid-base
- Redox
- Acid base plus redox
- Acid base and/or redox
Answers
Corrosive chemical typically involve either acid-base reactions or redox reactions, and sometimes both. Therefore the correct option is option D.
A corrosive chemical can interact with a substance in an acid-base reaction by either giving or receiving protons, which can harm the substance.
For instance, powerful acids that react with the metal to produce hydrogen gas, such as hydrochloric acid, can corrode metals. Redox reactions include the transfer of electrons from or to a substance by corrosive chemicals, which causes the substance to degrade.
For instance, iron rusts because iron oxidises, which occurs when iron loses electrons to oxygen in the presence of water. Some caustic substances can also conduct redox as well as acid-base reactions. For instance, sulfuric acid can corrode metals by oxidising the metal and causing an acid-base interaction. Therefore the correct option is option D.
For such more question on chemical:
https://brainly.com/question/29886197
#SPJ11
There are 120.0 mL of O2 at 700. 0 mmHg and 15⁰ C. What is the number of grams present?
Answers
Answer:
0.1498 g of O2.
Explanation:
The Behavior of Gases => Ideal Gas Law.
The ideal gas law is a single equation that relates the pressure, volume, temperature, and the number of moles of an ideal gas, which is:
\(PV=nRT,\)where P is pressure in atm, V is the volume in liters, n is the number of moles, R is the ideal gas constant (0.082 L*atm/mol*K), and T is the temperature in the Kelvin scale.
So we have to convert pressure from 700.0 mmHg to atm, volume from 120.0 mL to L, and 15 °C to K.
Let's convert pressure taking into account that 1 atm equals 760 mmHg, like this:
\(700.0\text{ mmHg}\cdot\frac{1\text{ atm}}{760\text{ mmHg}}=0.9211\text{ atm.}\)Remember that 1 L equals 1000 mL, so 120.0 mL would be equal:
\(120.0\text{ mL}\cdot\frac{1\text{ L}}{1000\text{ mL}}=0.1200\text{ L.}\)And the conversion from °C to K is just sum °C with 273, so 15 °C in K is:
\(K=\degree C+273=15\degree C+273=288\text{ K.}\)Finally, we can use the ideal gas formula, solving for 'n' (number of moles) and replacing the data that we have, as follows:
\(\begin{gathered} n=\frac{PV}{RT}, \\ \\ n=\frac{0.9211\text{ atm}\cdot0.1200\text{ L}}{0.082\frac{L\cdot atm}{mol\cdot K}\cdot288\text{ K}}, \\ \\ n=4.680\cdot10^{-3}\text{ moles.} \end{gathered}\)Now, the final step is to convert 4.680 x 10⁻³ moles of O2 to grams using the molar mass of O2 that can be calculated using the periodic table, which is 32 g/mol. The conversion will look like this:
\(4.68\cdot10^{-3\text{ }}moles\text{ O}_2\cdot\frac{32\text{ g O}_2}{1\text{ mol O}_2}=0.1498\text{ g O}_2.\)The answer would be that there are 0.1498 g of O2.
HELP!!! WILL MARK BRAINLIEST!!!
Which of the following metals is paramagnetic?
Question 5 options:
A)
Calcium
B)
Magnesium
C)
Sodium
D)
Beryllium
Answers
Answer:
A , B, C
Explanation: D is a Diamagnetic
The metals that are paramagnetic are Calcium, Magnesium, and Sodium. The correct options are A, B, and C.
What is paramagnetism?A weak magnetic field supplied externally can weakly attract some materials, which then create internal magnetic fields that are directed in the same direction as the applied magnetic field. This phenomenon is known as paramagnetism.
Despite not having an unpaired electron, the metal magnesium exhibits paramagnetic behavior. Sodium is also paramagnetic.
Atoms that have their electrons coupled are said to be diamagnetic, whereas those that don't are said to be paramagnetic. However, despite having no free electrons, calcium is said to be paramagnetic.
Thus, the correct options are A, Calcium, B, Magnesium, and C. Sodium.
To learn more about paramagnetism, refer to the link:
https://brainly.com/question/28216590
#SPJ2
The temperature of a sample of water increases from 22.5°C to 85.7°C. It absorbs
7540 J of heat. The mass of the water is
3.51g
119g
28.5g
0.0351g

Answers
We can answer this question using the formula: q=mcΔT
q = heat (joules, J)
m = mass (g)
c = specific heat capacity (J/g°C)
ΔT = change in temperature (temp final - temp initial) in °C
Specific heat capacity: the amount of energy required to increase the temperature of 1 g of an object by 1°C
the specific heat capacity of water is 4.18 J/g°CUsing the information and isolating for m:
q = 28.5 g
A student cuts out fins in a paper cup and hangs the cup on a metal stand. The student plans to place the metal stand by a windowsill and expects the paper cup to rotate as the wind blows. What kind of energy does the cup possess before the student places the metal stand by a windowsill?
A chemical energy
B elastic energy
C heat energy
D potential energy

Answers
Answer: Potential energy
Explanation:
Potential energy is exactly what it sounds like, it has the potential to produce energy. The paper cup hanging on a metal stand has potential energy, it is not currently producing energy, but it has the potential to.
Chemical energy is caused by a chemical reaction. Elastic energy is caused by strain, such as a rubber band being pulled and held in that pulled position. Heat energy is caused by heat, or something heating up.
Potential energy is the only thing that fits these criteria.
P4 + 6Cl2 → 4PCl2
If 5 moles of P4 reacted with 22 moles Cl2 according to the above reaction, determine:
a) How many moles PCl 3 are produced
b) How many moles of P 4 are left in excess after the reaction (if any)
c) How many moles of Cl 2 are left in excess after the reaction (if any)
Answers
According to the stoichiometry of the given chemical equation, 20 moles of PCl₃ are produced with no moles of P₄ and Cl₂ left.
What is stoichiometry?It is the determination of proportions of elements or compounds in a chemical reaction. The related relations are based on law of conservation of mass and law of combining weights and volumes.
Stoichiometry is used in quantitative analysis for measuring concentrations of substances present in the sample.
In the given reaction , 1 mole of phosphorous gives 4 moles of PCl₃, thus, 5 moles of phosphorous gives 5×4/1=20 moles of PCl₃ and no moles of P₄ and Cl₂ left.
Learn more about stoichiometry,here:
https://brainly.com/question/9743981
#SPJ1
How much heat must be transferred to 55 g of ice to change the ice's
temperature from -13°C to -5.0°C? (The specific heat capacity of ice is 2.11
J/g.°C)
Answers
Q= m x c x t
M= 55 g
C=2.11
T=8
Pls help me if your good at science:)remember I need two answer choices


Answers
A has a force hitting the ball
D is changing the force when the ball hits the net
Answer:
A and D, my bad
Explanation:
If an object goes through a chemical reaction but does not change in mass that object has
Answers
Answer:
The law of conservation of mass
Explanation:
The law of conservation of mass
how many hydrogen atoms do you have four molecules of glucoes
Answers
Molecules of glucose (blood sugar) contain 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
Hope this helped :)
how many mercury atoms are contained in 0.37 moles of mercury?
Answers
Complete and balance the equation for the reaction of sodium with water.
Na+_H2O+ H2
Answers
2Na + 2H2O → 2NaOH + H2
The balanced equation
what is the name of the chart that organizes elements in order of chemical properties?
Answers
The chart that organizes elements in order of their chemical properties is called the Periodic Table of Elements.
It is a tabular arrangement of elements based on their atomic number, electron configuration, and recurring chemical properties. The Periodic Table provides a systematic way to categorize and understand the properties of elements, as well as predict their behavior and relationships with other elements.
#SPJ11
Chemical equation for zinc carbonate and sulphuric vi acid
Answers
Answer: sulfuric acid + zinc carbonate → zinc sulfate + water + carbon dioxide
Explanation: In this type of reaction an acid reacts with a carbonate to give a salt, water and carbon dioxide. The reactants include elements which must also be present in the products.
Choose the response that would best complete (balance) the nuclear equation shown.

Answers
The response that would best complete (balance) the nuclear equation is the second option
e (zero up and -1 down)How to choose the correct optionThis is a nuclear reaction in which 3 hydrogen nuclei here each with 1 proton will combine to form a helium nucleus having 2 protons and 2 neutrons, and release one high energy electron also known as a beta particle
the reaction results to a release of an electron with zero mass number and -1 as the atomic number
Learn more about nuclear equation at
https://brainly.com/question/29863580
#SPJ1
Using the phase diagram for H₂O, what phase is water in at 1 atm pressure
and -5°C?
A. It is at its melting point.
B. It is in the gas phase.
C. It is in the liquid phase.
D. It is in the solid phase.
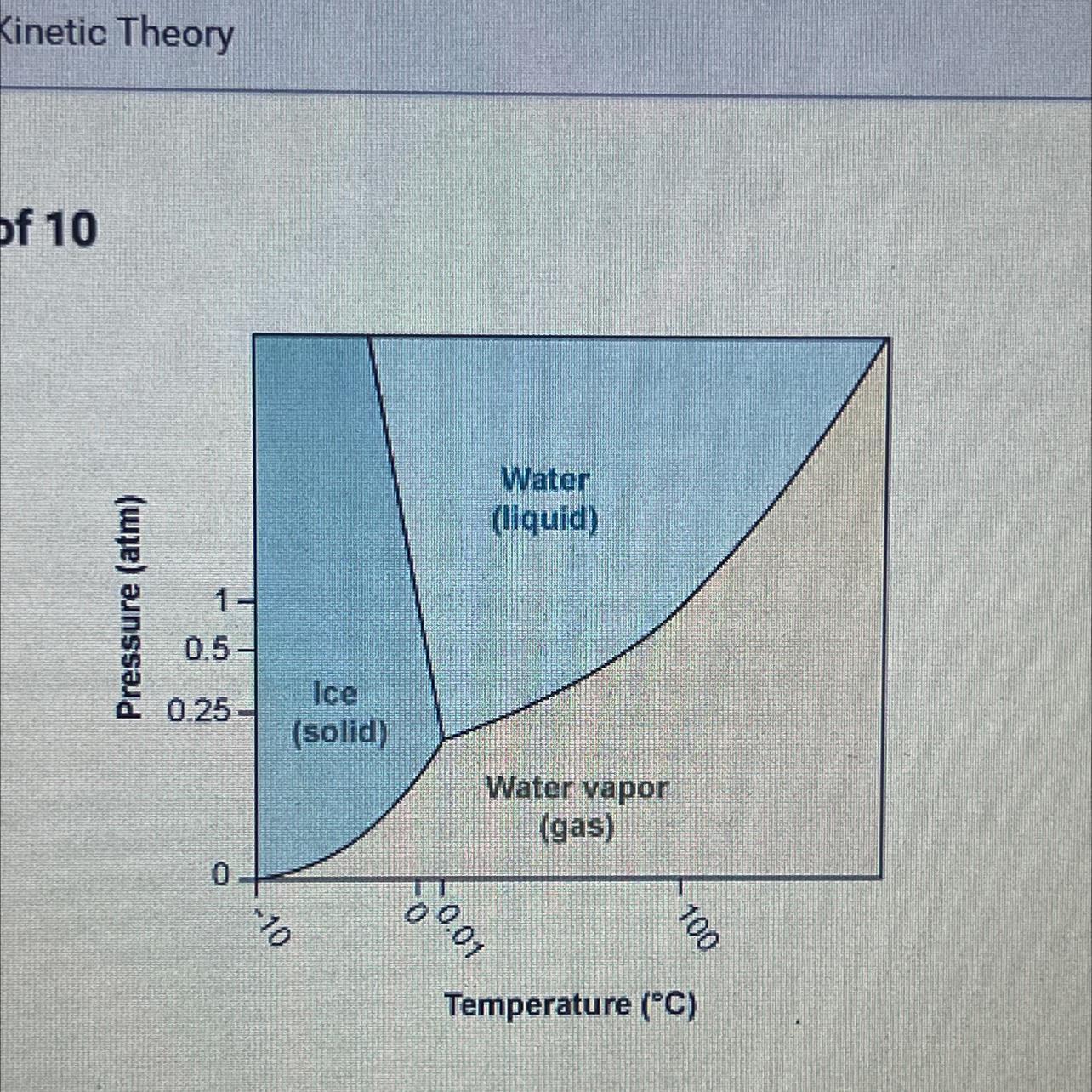
Answers
The number of phases that exist in equilibrium in a system depends upon the variables like temperature, pressure and composition. Here at 1 atm pressure water is in solid phase. The correct option is D.
What is phase diagram?The phase equilibria studies are made simpler by the use of plots which show how the various equilibria depend on the temperature, pressure and composition variables. These diagrams are called phase diagrams.
Here the pressure 1 atm on y-axis coincides with the temperature -5°C on x-axis. The water system is a one component system. So the water is in solid phase.
Thus the correct option is D.
To know more about phase diagrams, visit;
https://brainly.com/question/16945664
#SPJ9
why are hydrocarbons insoluble in water? a) the majority of their bonds are polar covalent carbon-to-hydrogen linkages. b) the majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages. c) they exhibit considerable molecular complexity and diversity. d) they are less dense than water.
Answers
Water is a polar molecule due to its bent molecular geometry and the electronegativity difference between oxygen and hydrogen.
The oxygen atom in water is more electronegative than hydrogen, resulting in an uneven distribution of charge within the molecule.Since the majority of hydrocarbon bonds are nonpolar, they do not readily interact with polar substances like water. The nonpolar nature of hydrocarbons leads to a lack of significant electrostatic interactions between the hydrocarbon molecules and water molecules. As a result, hydrocarbons are generally insoluble in water.It is important to note that option c) they exhibit considerable molecular complexity and diversity, and option d) they are less dense than water, are not accurate explanations for the insolubility of hydrocarbons in water. The solubility of hydrocarbons in water primarily depends on the polarity of the hydrocarbon molecules and their ability to interact with water molecules.
To know more about molecules visit :
https://brainly.com/question/32298217
#SPJ11
What happens in stage one of photosynthesis
Answers
Answer:
Photosynthesis occurs in two stages. During the first stage, the energy from sunlight is absorbed by the chloroplast. Water is used, and oxygen is produced during this part of the process.
Explanation:
Answer:
Chloroplast absorbs the energy emitted by the Sun.
_____________________________________________________
Context:
Photosynthesis:
The complex process by which carbon dioxide, water, and certain inorganic salts are converted into carbohydrates by green plants, algae, and certain bacteria, using energy from the sun and chlorophyll.
Chloroplast:
A plastid containing chlorophyll.
Chlorophyll:
The green coloring matter of leaves and plants, essentially to the production of carbohydrates by photosynthesis, and occurring in a blush-black form, C55J72MgN4O5 (chlorophyll a), and a dark-green form, C55H70MgtN4O6 (chlorophyll b).
Sun:
The star that is the central body of the solar system, around which the planets revolve and from which they receive light and heat: its mean distance form earth is about 93 million miles (150 million km), its diameter about 864,000 miles (1.4 million km), and it's mass about 330,000 times that of earth; its period of surface rotation is about 26 days at its equator but longer at higher latitudes.
Explanation:
There are two main phases in photosynthesis:
depending on light stage
Clause Cycle (AKA the dark stage or the light-independent stage)
In the light-dependent stage, ATP and NADPH are produced using photons, or light energy. Oxygen molecules start to form during this stage as a byproduct.
Utilizing the materials produced during the light-dependent stage, the Calvin cycle fixes CO2 molecules to produce carbohydrates (sugars). This stage can repeat itself indefinitely since RuBP, the initial chemical required for the stage to go further, also serves as its byproduct.
Name the unidentified species, and write each transmutation process in shorthand notation: (c) bombardment of ²³⁸U with a particle yields three neutrons and ²³⁹Pu.
Answers
unidentified species, and write each transmutation process in shorthand notation bombardment of ²³⁸U with a particle yields three neutrons and ²³⁹Pu.
What is neutrons ?The neutron is a subatomic particle that is slightly heavier than a proton and has a neutral charge (one that is neither positive nor negative). Atomic nuclei consist of protons and neutrons. Protons and neutrons are both referred to as "nucleons" because of how similarly they function inside the nucleus and because they both have masses that are about equal to one atomic mass unit. Nuclear physics explains their interactions and characteristics.
The arrangement of electrons around an atom's heavy nucleus largely determines its chemical characteristics. The atomic number, or number of protons, which determines the charge of the nucleus, determines the electron configuration. The neutron number is equal to the number of neutrons. Neutrons have no impact on the
To learn more about neutrons from the given link:
https://brainly.com/question/15120322
#SPJ4
Calcula la concentración porcentual de una disolución que se preparó disolviendo 30 g de Hidróxido de Litio en suficiente cantidad de agua para obtener 100 ml
Answers
Answer:
30 % m/V o 23 % m/m
Explanation:
Al leer el problema y entender el enunciado como que se usó suficiente cantidad de agua para obtener 100 mL, con la masa de LiOH podemos determinar la concentración en m/V.
m/V es una concentración que establece la relación de gramos de soluto en 100 mL de solución.
Como los gramos de soluto son 30g, la concentración en m/V es
30/100 = 0.3 % m/V
Podemos también establecer concentración m/m la cual establece la relación como masa de soluto en 100 g de solución. Como el volumen de solución coincide con el de solvente, la masa de solución la podemos obtener sumando masa de solvente + masa de soluto
30 g + 100 g = 130 g
Si tomamos el agua como solvente puro, y por densidad (1 g/mL) la masa de agua es 100g
Así el % m/m lo calculamos como (masa de soluto/masa solucion) . 100
(30g / 130g) . 100 = 23 % m/m
Jenna took an open bowl of leftover mashed potatoes from the refrigerator and noticed a difference in smell. She determined that chemical changes occurred since the potatoes were first placed there.
Which observations most likely led to Jenna’s conclusion?
Answers
Answer:
The change in smell
Explanation:
chemical reactions ccan lead to change in temperature, change in color and also change in smell