how many ml of 0.050 m cacn2 are needed to make 25.0 ml of 0.010 m solution? the molar mass of cacn2 is 80.11 g/mol.
1. 33.3 mL 2. 0.0188 mL 3. 30.0 mL 4. 12.0 mL 5. 7.50 mL 6. 83.3 mL 7. 63.0 mL
Answers
30.0 mL of 0.050 M Ca(CN)2 are needed to make 25.0 mL of 0.010 M solution. Hence, Volume of 0.050 M solution containing 0.00025 mol of Ca(CN)2= 0.00025 / 0.00125 = 0.2 L or 200 mL.
Molarity of Ca(CN)2 solution = 0.050 M Molarity of solution to be made = 0.010 MVolume of solution to be made = 25.0 mLNumber of moles of Ca(CN)2 in 25.0 mL of 0.010 M solution =0.010 * 25.0 / 1000 = 0.00025 molMolar mass of Ca(CN)2 = 80.11 g/mol
Mass of Ca(CN)2 in 0.00025 mol of Ca(CN)2 = 0.00025 * 80.11 = 0.020 m gNumber of moles of Ca(CN)2 in 0.050 M solution = 0.050 * 25.0 / 1000 = 0.00125 mol Therefore, Volume of 0.050 M solution containing 0.020 mg of Ca(CN)2 = (200/1000) * 0.020 = 0.004 mL or 4.0 mL Therefore, Volume of 0.050 M solution containing 20.0 mg of Ca(CN)2 = (4.0/0.020) * 20.0 = 400.0 mL or 0.400 L.
To know more about solution visit:
https://brainly.com/question/15757469
#SPJ11
Related Questions
express balanced equations for the production of nitric acid from nitrogen
Answers
There are several methods for producing nitric acid from nitrogen, but two common methods are:
1. Ostwald process:
4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)
2 NO(g) + O2(g) → 2 NO2(g)
3 NO2(g) + H2O(l) → HNO3(aq) + HNO2(aq)
Overall equation:
4 NH3(g) + 3 O2(g) + H2O(l) → 2 HNO3(aq) + 2 NO(g)
2. Birkeland-Eyde process:
N2(g) + 3 H2(g) → 2 NH3(g)
2 NH3(g) + 3 O2(g) → 2 NO(g) + 3 H2O(g)
2 NO(g) + O2(g) → 2 NO2(g)
3 NO2(g) + H2O(l) → 2 HNO3(aq) + NO(g)
Overall equation:
N2(g) + 3 O2(g) + 3 H2(g) → 2 HNO3(aq)
The end of a very long 5-mm-diameter rod is held at 124 C. The
surface of the rod is exposed to ambient air at 30 C, with a
convective heat transfer coefficient of 100 W/m2 K.
a) Determine the tempera
Answers
The end of a very long 5-mm-diameter rod is held at 124°C. The surface of the rod is exposed to ambient air at 30°C, with a convective heat transfer coefficient of 100 W/m2K. Determine the temperature at a radial distance of 2.5 mm from the rod's center. The thermal conductivity of the rod is 15 W/mK.b) What is the temperature gradient in the rod at this location?c).
What is the heat flux at this location?The temperature at a radial distance of 2.5 mm from the rod's center is 79.58°C.The solution for this problem can be found by following the steps below:Solution:a) The temperature of the rod, T, can be calculated using the formula for one-dimensional conduction:q/A = -k (d T/d r)whereq is the heat flux,A is the cross-sectional area of the rod,r is the radial distance from the center of the rod,k is the thermal conductivity of the rod,and T is the temperature of the rod.
Taking the boundary condition into account,T(r=0) = 124°CandT(r=2.5 mm) = 30°C, the solution to the differential equation is:T = T0 + (T1 - T0) (r/R)2whereT0 = 30°CT1 = 124°CR = 2.5 mm/2 = 1.25 mmso,T = 30 + (124 - 30) (r/1.25)2 = 30 + 78 (r/1.25)2at r = 2.5 mm,T = 79.58°Cb) The temperature gradient, d T/d r, is given by the derivative of the above equation:d T/d r = 124 (r/1.25)2 / 1.25where d T/d r = 98.72°C/mat r = 2.5 mmc) The heat flux, q/A, is given by the Fourier's law of heat conduction:q/A = -k (d T/d r)whereq/A = -15 (98.72/1000) = -1.48 W/m2at r = 2.5 mmTherefore, the temperature at a radial distance of 2.5 mm from the rod's center is 79.58°C, the temperature gradient in the rod at this location is 98.72°C/m, and the heat flux at this location is -1.48 W/m2.
Learn more about diameter:
https://brainly.com/question/30460318
#SPJ11
what is the mass of 1 mole of water
Answers
Answer:
18.01528 g/mol
Explanation:
Using the equation to figure this out
Answer:
18.01528 g/mol
Which of these would you MOST likely find in a country with an unlimited government?
A checks and balanceschecks and balances
B one person ruleone person rule
C protection of free speechprotection of free speech
D separation of powers
Answers
Explanation: I got it right
3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many moles of water can be made when 146.3 grams of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element
Molar Mass
Hydrogen
1
Nitrogen
14
Copper
63.5
Oxygen
16
Answers
The given chemical equation is: 3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O1Oxygen16Now, let us balance the given chemical equation by following the Law of Conservation of Mass.Balancing the chemical equation: 3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O1Oxygen16Number of atoms on both sides of the chemical equation should be the same. Let's check one by one.Number of Copper (Cu) atoms:
On the left side: 3 atomsOn the right side: 3 atomsNumber of Nitrogen (N) atoms:On the left side: 24 atoms (8 × 3)On the right side: 6 atoms (2 × 3)Number of Oxygen (O) atoms:On the left side: 24 atoms (8 × 3)On the right side: 24 atoms (2 × 3 + 4 × 4 + 16)All the elements on both sides are equal, so the given chemical equation is balanced.The chemical reaction is a redox reaction in which Copper acts as a reducing agent while Nitric Acid acts as an oxidizing agent. Redox reaction is a type of chemical reaction in which both oxidation and reduction processes occur simultaneously. In the given chemical equation, Copper (Cu) gets oxidized to Copper Nitrate [Cu(NO3)2] by Nitric Acid (HNO3) by donating electrons. It loses two electrons to form Cu(NO3)2.Cu → Cu2+ + 2e- (oxidation)On the other hand, Nitric Acid (HNO3) gets reduced to Nitrogen Monoxide (NO) by accepting electrons. It gains two electrons to form NO.4H+ + 2NO3- + 8e- → 2NO + 4H2O (reduction)Thus, Copper (Cu) acts as a reducing agent while Nitric Acid (HNO3) acts as an oxidizing agent in the given redox reaction.For such more question on chemical equation
https://brainly.com/question/26694427
#SPJ8
what is the energy that travels from sun in the form of waves
Answers
Answer:
longwaves or solar radiation
Answer:
Solar Radiation
Explanation:
There could actually be different ways to say this anwser. This was just how I leaned It In school! :)
Atoms in a liquid have _______________ energy than atoms in a solid, so the easiest way to change a solid to a liquid is to add ________________. When changing from a solid to a liquid, there is a magic temperature for every substance called the ______________________________.
Answers
Answer
Atoms in a liquid have more kinetic energy
so the easiest way to change a solid to a liquid is to add heat
When changing from a solid to a liquid, there is a magic temperature for every substance called the boiling point
Examine the diagram shown. Which are the correct labels for continental plates 1 and 2?
2
equator
1
1 = Antarctic, 2 = African
1 = Indo-Australian, 2 = Eurasian
1 = North American, 2 = Pacific
1 = South American, 2 = Antarctic
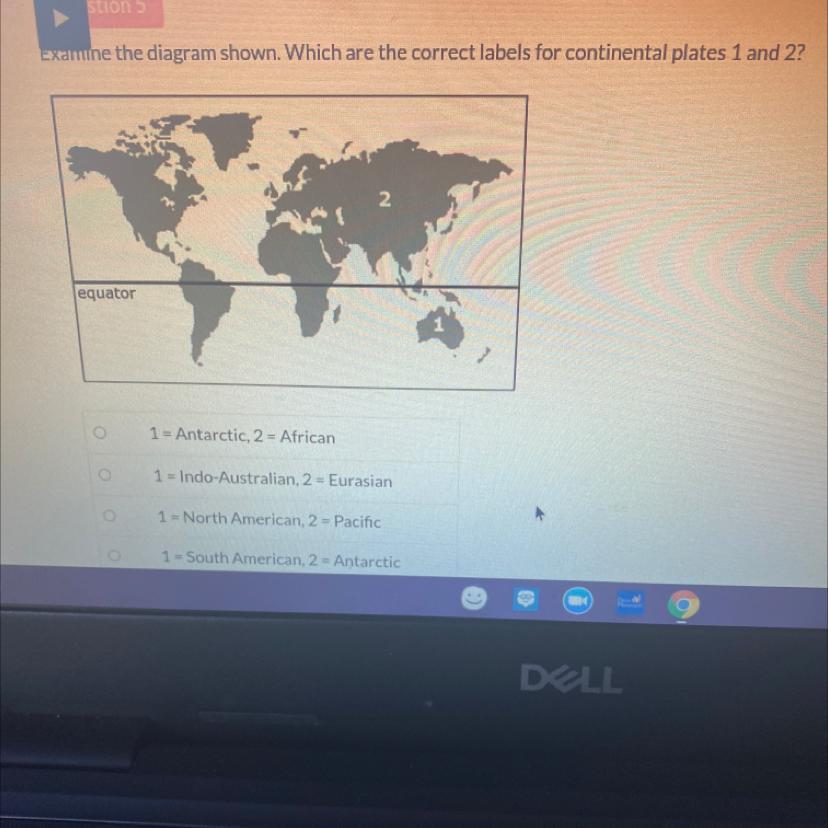
Answers
Answer:
Australian and eurasian
Explanation:
Aluminum is often used in the structure of light-weight bicycle frames. How many
atoms of aluminum are in 20.00 g of Al?
Answers
Answer:
The first thing that you need to do here is to figure out the mass of the sample.
To do that, you can use its volume and the fact that aluminium is said to have a density of
2.702 g cm
−
3
, which implies that every
1 cm
3
of aluminium has a mass of
2.702 g
.
Explanation:
2H2O(g) -- 2H2(g) + O2(g)
What total volume of gas (at STP) is produced by the electrolysis of 4 moles of H2O?
Answers
Explanation:
\(v = vdm \times n\)
Vdm=22.4dm.
mole(n)=4 mol
therefore the total volume
\(v = 22.4 \times 4 \\ v = 89.6dm\)
How do insecticides cause chemical pollution?
Answers
Pesticides have the potential to pollute lawn, water, and other vegetation. Pesticides can be poisonous to a variety of different organisms in addition to insects and weeds, such as birds, fish, helpful insects, and non-target plants.
Do insecticides contribute to air pollution?Pesticides release a variety of pollutants, including volatile organic compounds and hazardous air pollutants (HAPs) (VOC). These contaminants may cause health issues that have an impact on the neighbourhood, the community, and the locals.
What leads to the contamination of harmful chemicals?Emissions from coal-fired power plants, businesses, and refineries, as well as those from automobiles, trucks, and buses, are the main sources of harmful air pollution outside. Hazardous air pollutants from sources like solvents, asbestos-containing building materials, and cigarette smoke can also be found in indoor air.
To know more about Pesticides visit:-
brainly.com/question/12284986
#SPJ9
a sample of gas at a constant temperature has an initial pressure reading of 2.00 atm with a volume of 175.0 ml. after a change in pressure, the final volume reading is 550.0 ml. calculate the final pressure.
Answers
The final pressure of the gas is 0.63 atm which has an initial pressure reading of 2.00 atm with a volume of 175.0 ml.
Given data in the question is:
Initial pressure- 2.00 atm
Initial volume- 175 ml
Final volume- 550 ml
The final pressure is supposed to be calculated,
As per the equation of the Boyle's law that we know:
P1V1=P2V2
Substituting the given data in the equation above:
(2)(175)=P2(550)
P2=(2)(175)/550
P2=0.6363 atm
Boyle's law states " The pressure of gas is inversely proportional to the volume occupied by the gas when the temperature(in kelvins) is held constant ".
The final pressure of the gas is 0.63 atm.
Learn more about Boyle's Law:
brainly.com/question/1437490
#SPJ4
Describe how magnesium nitrate crystals can be obtained from a solution
Answers
Answer:
magnesium carbonate reacts with aqueous acids to release carbon dioxide and water
MgCO3 + 2 HCl → MgCl2 + CO2 + H2O.
How to -
Step 1: Reaction
- Leave the dilute hydrochloric acid in a beaker.
- Add Magnesium carbonate slowly until it is in excess or until no more gas seem to be getting liberated.
Step 2: Filtration
- Filter with filter paper and funnel.
- Filter off the excess magnesium carbonate as magnesium chloride will be in aqueous form (liquid) and will come out with the filtrate. The residue is the excess magnesium carbonate.
Step 3: Crystallization to obtain solid crystals from the filtrate.
- Pour filtrate solution into evaporating dish/basin
- Provide heat using Bunsen burner
- Pour solution into an evaporating basin and heat over a water bath
- Stop heating when crystals start to form
allow water to evaporate until pure crystals remain.
- Dry crystals using absorbent paper or warm oven.
Precautions
- Use personal protective equipment such as gloves, a lab coat and wear eye protection, especially when heating.
- Avoid inhaling unnecessary gases during the whole process.
Mark me as brainliest
3) The distance from the Earth to the Moon is 238,900 mi. What is the distance in inches?
Answers
The distance from the Earth to the Moon in inches is 18304704000 inches.
What is the distance from the Earth to the Moon?Distance is a measure of the point of separation of two point or the amount of space between two points.
The SI unit for measuring distance is meters with symbol m.
Other common units of measuring distance or length include:
miles with distance miinches with symbol infeet with symbol ftThe distance from the Earth to the Moon is given in miles as 238,900 mi
1 mile = 63360 inches
238900 miles = 238900 * 63360
238900 miles = 18304704000 inches.
Distance = 18304704000 inches.
Learn more about miles to inches at: https://brainly.com/question/93330
#SPJ1
What are land plants used for?
Answers
Answer:
Land plants are also known as Embryophytes. Embryophytes are complex multicellular eukaryotes with specialized reproductive organs. The name derives from their innovative characteristic of nurturing the young embryo sporophyte during the early stages of its multicellular development within the tissues of the parent gametophyte.
Explanation:
Answer:
there used for mountains , wait I'm just trynna get points ya feel me
Explanation:
Which of the following is a salt that could be generated by combining a weak acid and a weak base? Select the correct answer below: O NaCl Na,SO4 O NH,NO 443 NH F
Answers
The right answer is NH4F.
A salt can be defined as any ionic compound that is composed of positively charged cations and negatively charged anions. A weak acid is an acid that partially dissociates in water to create a relatively little number of hydrogen ions. A weak base is a base that does not completely dissolve in water or only partially ionizes to release hydroxide ions. By reacting a weak acid with a weak base, a salt can be generated.
NH4F is the correct answer because NH4+ is a weak acid and F- is a weak base. When NH4+ is combined with F-, NH4F is formed. NH4F is ammonium fluoride, which is an ionic salt that is made up of ammonium cations (NH4+) and fluoride anions (F-).
To know more about Ammonium Fluoride visit:
https://brainly.com/question/20528587
#SPJ11
PLEASE HELP Which of these could DNA determine?
1. the pattern of a tiger’s stripes
2. the borders of a country
3.the number of dandelions in a field
4.the elements that bond to form water
Answers
Answer:
1. The pattern of a tiger's stripes
Explanation:
DNA is the code within a living organism that tells how it will look and how it will behave structurally. The only one of these choices that fits that is 1.
DNA helps in determining pattern of tiger's stripes.As DNA gives qualitative relationship about inherited characteristics and tiger's stripes. Thus,option A is correct.
What is DNA?
It is a polymer made of two polynucleotide chains coiled around together giving DNA a helical structure. It carries all genetic information and is responsible for growth ,functioning and reproduction.
Both strand of DNA store same biological information .Information is replicated when the two strands begin to separate.A large part of DNA is non-coding meaning they do not serve as protein sequences.
DNA is organized into chromosomes which are large structures. These chromosomes are duplicated in the process of DNA duplication.The human genome comprises of 3 billion base pairs of DNA which are arranged to give rise to 46 chromosomes.
Therefore,DNA determines pattern of tiger's stripes.
To know more about DNA :
https://brainly.com/question/264225
#SPJ2
A sample of sulfur has a mass of 32.066 grams. How many moles of sulfur
are present in the sample? *
Answers
Answer:
1 mole
Explanation:
Given parameters:
Mass of sulfur = 32.066g
Unknown:
Number of moles of sulfur = ?
Solution:
The number of moles of sulfur can be determined using;
Number of moles = \(\frac{mass}{molar mass}\)
Molar mass of sulfur = 32g/mol
Number of moles = \(\frac{32.066}{32}\) = 1 mole
can someone help me solve the questions below using the data table below PLEASEE
DATA TABLE:
Mass of flask and vinegar solution- 25.17g
Mass of flask- 15.12g
Volume of vinegar solution (in mL)- 10.00ml
Initial volume of NaOH (in mL)-0.00ml
Final volume of NaOH (in mL)-39.00ml
CALCULATIONS:
Mass of vinegar solution- 10.0503g
Volume of NaOH used in titration (in mL)-39.00ml

Answers
The molarity of NaOH solution is 0.114 M.
Given, Mass of flask and vinegar solution= 25.17 g.Mass of flask= 15.12 gVolume of vinegar solution (in mL)= 10.00 mlInitial volume of NaOH (in mL)= 0.00 mlFinal volume of NaOH (in mL)= 39.00 mlThe Mass of vinegar solution is 10.0503 g.The volume of NaOH used in titration is 39.00 ml.Let's calculate the molarity of the NaOH solution.First, calculate the moles of NaOH used in the reaction. Moles of NaOH = Molarity × Volume of NaOH (in L) Converting volume in mL to L,Volume of NaOH used = 39.00 mL = 39.00/1000 L = 0.0390 LThe molarity of NaOH solution is given by;Molarity of NaOH = Moles of NaOH / Volume of vinegar solution (in L)Converting volume in mL to L,Volume of vinegar solution = 10.00 mL = 10.00/1000 L = 0.0100 LNow, substituting the values; Molarity of NaOH = 0.114 M.
for such more questions on molarity
https://brainly.com/question/30404105
#SPJ8
Definition: This is an important principle of the scientific method in which an experiment can accurately be reproduced by an independent researcher. Replication shows that test results can be reproduced with different scientists and lab equipment.
Answers
Answer:
Replicate.
Explanation:
Replicate: This is an important principle of the scientific method in which an experiment can accurately be reproduced by an independent researcher. Replication shows that test results can be reproduced with different scientists and lab equipment.
Hence, when test experiments are performed or conducted and the same results are gotten, it ultimately implies that the research is statistically significant and correct. A theory can be developed by the repetition or replication of a hypothesis.
In scientific process, researchers do not rely on a single test. They replicate tests over and over to see if they will get the same results.
In conclusion, if an experiment, investigation or findings can't be replicated then the method of testing isn't sufficient, theoretical and not reliable for use.
Answer:
Repitition, not replication
Explanation:
Which of these mixtures is a suspension?
A. salad dressing
B. Gelatin
C. whipped cream
D. apple juice
Answers
Answer:
D. apple juice
Explanation:
How can you show using Pauli's exclusion principle that p sub shell can have only 6 electrons?

Answers
where l = subshell value.
"l"values of subshell are.
s = 0.
p = 1.
d = 2.
f = 3.
So in p orbital we have 6 electrons.
How many protons are in the nucleus of a lithium atom in its excited state
Answers
Answer:
the answer is three protons
When a lamp that is rated 500W at 115V is connected to a 120V power supply, the current of the circuit will be _______ Amps.
Answers
To calculate the current (I) in the circuit, we can use Ohm's Law, which states that the current is equal to the voltage divided by the resistance. In this case, the resistance is not directly given, but we can calculate it using the power (P) rating of the lamp.
The power (P) can be calculated using the formula:
P = V * I
Rearranging the formula, we get:
I = P / V
Substituting the given values:
P = 500 W
V = 115 V
I = 500 W / 115 V
I ≈ 4.35 Amps
Therefore, when the lamp rated at 500W and 115V is connected to a 120V power supply, the current in the circuit will be approximately 4.35 Amps.
Learn more about voltage here:
https://brainly.com/question/32002804
#SPJ11
what is periodic table
Answers
Answer:
the periodic table is the table of contents of the elements
an al2o3 whisker is a small single crystal used to reinforce metal-matrix composites. given a cylindrical shape, calculate the number of al atoms and the number of o atoms in a whisker with a diameter of 1 mm and a length of 25 mm. (the density of al2o3 is 3.97 mg>m3 .)
Answers
The \(Al_2O_3\) whisker with a diameter of 1 mm and a length of 25 mm contains approximately \(1.528 * 10^{-13}\) aluminum (Al) atoms and \(2.292 * 10^{-13}\) oxygen (O) atoms.
To calculate the number of Al (aluminum) atoms and O (oxygen) atoms in the \(Al_2O_3\) whisker, we will first calculate the volume of the whisker using its dimensions. The volume of a cylinder is given by the formula
\(V = \pi r^2h\),
where r is the radius and h is the height (length) of the cylinder. Given that the diameter of the whisker is 1 mm, we can calculate the radius by dividing the diameter by 2:
Radius (r) = 1 mm / 2 = 0.5 mm = \(0.5 * 10^{-3} m\)
The length of the whisker is 25 mm = \(25 * 10^{-3} m\)
Plugging these values into the formula for the volume of a cylinder, we get: \(V = \pi \left(0.5 \times 10^{-3}\right)^2 \times 25 \times 10^{-3} \, \text{m}^3\)
Next, we will calculate the mass of the whisker using its density. The formula to calculate the mass is given by:
Mass = Density * Volume
Given that the density of \(Al_2O_3\) is \(3.97 mg/m^3\),
we convert it to \(kg/m^3\) by dividing by 1000:
Density = \(3.97 mg/m^3 = 3.97 * 10^{-6} kg/m^3\)
Now we can calculate the mass of the whisker:
Mass = \(3.97 * 10^{-6} kg/m^3 * V\)
Finally, we can calculate the number of atoms using Avogadro's number (\(6.022 * 10^{23}\) atoms/mol).
Since the molar mass of \(Al_2O_3\) is 101.96 g/mol,
we can calculate the number of moles:
Number of moles = Mass (in kg) / Molar mass (in g/mol)
Number of atoms = Number of moles * Avogadro's number
Since there are 2 Al atoms and 3 O atoms in 1 molecule of \(Al_2O_3\) , we can calculate the number of Al atoms and O atoms in the whisker:
Number of Al atoms = Moles of \(Al_2O_3\) × 2
Number of Al atoms ≈ \((7.641 * 10^{-14} mol) * 2\)
Number of Al atoms ≈ \(1.528 * 10^{-13}\)
Number of O atoms = Moles of \(Al_2O_3\) × 3
Number of O atoms ≈ \((7.641 * 10^{-14} mol) * 3\)
Number of O atoms ≈ \(2.292 * 10^{-13}\)
Therefore, the \(Al_2O_3\) whisker with a diameter of 1 mm and a length of 25 mm contains approximately \(1.528 * 10^{-13}\) aluminum (Al) atoms and \(2.292 * 10^{-13}\) oxygen (O) atoms.
To learn more about atoms ,
https://brainly.com/question/6258301
#SPJ4
How many moles are there in 5g of faraday
I am to submit this now
Please help with this ASAP
Answers
Faraday is a unit of electric charge, not a substance. It is equivalent to the charge on one mole of electrons, which is approximately 96,485 coulombs. Therefore, the number of moles in a certain amount of Faraday is not applicable as it is a unit of charge, not of substance.
What is faraday?Faraday is a unit of electric charge named after the British scientist Michael Faraday. It is defined as the amount of electric charge that is transported by a steady current of one ampere in one second. The Faraday is equivalent to the charge on Avogadro's number of electrons, approximately 6.022 x 10^23 electrons. The Faraday is often used in electrochemistry to express the magnitude of electric charge involved in chemical reactions. For example, in the electrolysis of water, the number of Faradays of charge required to produce a certain amount of hydrogen or oxygen is often measured.
Learn more about faraday in brainly.com/question/1640558
#SPJ1
consider the compounds cl2, hcl, f2, naf, and hf. which compound has a boiling point closest to that of argon? explain.
Answers
The compound that has a boiling point closest to that of Argon is HF. This is because HF has the strongest intermolecular forces (hydrogen bonding) among the given compounds.
The boiling point of a compound depends on the strength of the intermolecular forces that exist between the molecules. The stronger the intermolecular forces, the higher the boiling point.
The weaker the intermolecular forces, the lower the boiling point. The boiling point of Argon is -186°C. Out of the given compounds, the boiling point of HF is the closest to the boiling point of Argon.
The boiling point of HF is -83.8°C. This is because HF has hydrogen bonding which is the strongest intermolecular force among the given compounds. The other compounds such as Cl2, F2, HCl, and NaF, have weaker intermolecular forces than HF. Therefore, they have a lower boiling point than HF.
Learn more about HF here:
https://brainly.com/question/14581360#
#SPJ11
please help :) thanks

Answers
Explanation:
it is the one you have selected because it is the only solid one
Students want to conduct a new investigation using a larger bag of water. Using Table 1,
predict the weight of 300 grams of water after 24 hours.
Answers
We can actually deduce here that in the new investigation, based on the data, the weight of 300 grams of water after 24 hours is predicted to be 299.3 grams.
How we arrived at the above solution?Water evaporation is the cause of the weight loss. Water transforms from a liquid to a gas through evaporation. Temperature, humidity, and wind are a few of the variables that have an impact on the rate of evaporation.
There was no wind, a humidity of 50%, and a temperature of 25°C during the experiment. It is anticipated that part of the water will evaporate during the period of 24 hours due to the favorable evaporation conditions present.
Learn more about investigation on https://brainly.com/question/27960054
#SPJ1