How many grams of N2 gas are present in a 20.0 L tank of N2 that is at 290 K and 2.75 atm?
Answers
Considering the ideal gas law, 64.68 grams of N₂ gas are present in a 20.0 L tank of N₂ that is at 290 K and 2.75 atm.
Ideal gas lawIdeal gases are a simplification of real gases that is done to study them more easily. It is considered to be formed by point particles, do not interact with each other and move randomly. It is also considered that the molecules of an ideal gas, in themselves, do not occupy any volume.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P×V = n×R×T
where:
P is the gas pressure.V is the volume that occupies.T is its temperature.R is the ideal gas constant. The universal constant of ideal gases R has the same value for all gaseous substances.n is the number of moles of the gas. Mass of N₂In this case, you know:
P= 2.75 atmV= 20 LT= 290 KR= 0.082 \(\frac{atmL}{molK}\)n= ?Replacing in the ideal gas law:
2.75 atm× 20 L = n×0.082 \(\frac{atmL}{molK}\)× 290 K
Solving:
n= (2.75 atm× 20 L)÷ (0.082 \(\frac{atmL}{molK}\)× 290 K)
n= 2.31 moles
Being the molar mass of N₂ 28 g/mol, then the mass of 2.31 moles is calculated as:
mass= 28 \(\frac{g}{mole}\)× 2.31 moles
Solving:
mass= 64.68 grams
Finally, 64.68 grams of N₂ gas are present in a 20.0 L tank of N₂ that is at 290 K and 2.75 atm.
Learn more about the ideal gas law:
https://brainly.com/question/4147359
#SPJ1
Related Questions
Which statement is true of a mechanical wave? a It must have a medium.
b It can travel in a vacuum.
c It must travel in empty space.
d Both sound and light are examples.
Answers
Answer:
The answer is A. It must have a medium
Explanation:
With out a medium the wave will not travel at all.
-Hope This Helps!
-Justin:)
it needs a medium to travel
please answer of the questions

Answers
Succession is the process of incremental alterations in the make-up of an ecological community over time. Living things occupy exposed and newly formed rock initially in primary succession.
Which is a succession example?For instance, following a forest fire that destroys all the adult trees in a certain terrain, grasses may develop, then shrubs and other tree species, until ultimately the pre-fire community is once more present. Following an interruption, such as a fire, secondary succession starts.
What does the succession process entail exactly?The term "successful" is used to refer to the process of obtaining a licence to practise law in a particular jurisdiction. There are mosses or lichens in the area where earliest species to exist. Paraphrase: They prepare that soil such that larger species, and ultimately trees, may grow there. Paraphrase: they prepare the soil.
To know more about initially visit:
https://brainly.com/question/3256540
#SPJ1
A chemist has one solution that is 20% acid and a second that is 65% acid. How many gallons of each should be mixed together to get 120 gallons of a solution that is 50% acid?
Answers
To get 120 gallons of a solution that is 50% acid, the chemist should mix 60 gallons of the 20% acid solution with 60 gallons of the 65% acid solution.
To determine the quantities of the two solutions needed, we can set up an equation based on the acid content and the total volume of the solution. Let's assume x represents the amount (in gallons) of the 20% acid solution and y represents the amount (in gallons) of the 65% acid solution.
Since the total volume of the final solution is 120 gallons, we have the equation:
x + y = 120 --- Equation 1
Next, we need to consider the acid content in the mixture. The acid content in the 20% acid solution is 20% of x, while the acid content in the 65% acid solution is 65% of y. The acid content in the final solution should be 50% of the total volume (120 gallons), so we have another equation:
(20/100) * x + (65/100) * y = (50/100) * 120 --- Equation 2
Simplifying Equation 2, we get:
0.2x + 0.65y = 60 --- Equation 3
Now, we can solve the system of equations formed by Equations 1 and 3 to find the values of x and y. By solving the equations, we find that x = 60 and y = 60. This means that the chemist should mix 60 gallons of the 20% acid solution with 60 gallons of the 65% acid solution to obtain 120 gallons of a solution that is 50% acid.
Therefore, the chemist should mix 60 gallons of the 20% acid solution with 60 gallons of the 65% acid solution to obtain the desired solution.
Learn more about acid
brainly.com/question/29796621
#SPJ11
CaCo3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> CaO (s) + CO2 (g)
a. 178.3 kJ
b. 571.8 kJ
C. -1029 kJ
d. -2236 kJ

Answers
Answer:Typically a decomposition reaction is endothermic. Use the decomposition of limestone (CaCO3) to draw an energy diagram for a decomposition reaction assuming it occurs at constant pressure.
Explanation:hope this helps
When a small piece of copper metal is added to a silver nitrate solution, the following reaction occurs: 2Ag+NO3+Cu → Cu (NO3)2+2Ag
This equation represents both a single replacement reaction AND a(n) ______________________ reaction.
Question 4 options:
A. oxidation - reduction
B. neutralization
C. combustion
D. decomposition
Answers
Answer:
A. Oxidation-reduction
Explanation:
I assume you mean the reaction is:
Ag2NO3(aq) + Cu(s) -> 2Ag(s) + Cu(NO3)2(aq)
Either way:
Solids have the oxidation number of 0. So in the beginning of the reaction Cu(s) has the oxidation number 0, and at the end it has a oxidation number of +2. So it was oxidized.
Ag in the beginning of the reaction has the oxidation number of +1, and ends with the oxidation number of 0. It was reduced.
So its an oxidation reduction.
4. Which of the following terms best describes the reaction?A) allostericB) exergonicC) endergonicD) anabolicE) nonspontaneous5. Which of the following would be the same in an enzyme-catalyzed or -uncatalyzedreaction?A) A B) B C) C D) D E) E

Answers
4. The answer is B, the reaction is exergonic because DG < 0, meaning the reaction is spontaneous and energy is released.
5. The answer is D, even if the reaction is catalysed, D will not change it will remain the same because the beginning and the end of the reaction will not change.
A sample of fluorine gas at STP contains 4.088x10^24 atoms. What is the volume in liters of the sample?
Record your answer to 1 decimal place. Do not put units on your answer.
Answers
According to the concept of Avogadro's number and STP conditions volume in liters of the sample is 15,388.7.
What is Avogadro's number?Avogadro's number is defined as a proportionality factor which relates number of constituent particles with the amount of substance which is present in the sample.
According to the definitions, Avogadro's number depend on determined value of mass of one atom of those elements.It bridges the gap between macroscopic and microscopic world by relating amount of substance with number of particles.
Number of atoms can be calculated using Avogadro's number as follows: mass/molar mass×Avogadro's number
Number of moles is obtained as, number of atoms/Avogadro's number=4.088×10²⁴/6.023×10²³=6.78 moles on substitution in formula of PV=nRT=V=6.78×8.314×273/1=15,388.7 liters.
Thus, the volume is 15,388.7 liters of the sample.
Learn more about Avogadro's number,here:
https://brainly.com/question/11907018
#SPJ1
The average the temperature of the planets
Answers
Answer: Mercury - 800°F (430°C) during the day, -290°F (-180°C) at night. Venus - 880°F (471°C) Earth - 61°F (16°C) Mars - minus 20°F (-28°C)
Explanation: hope this helps!
THEORY Ia. What is the relationship between elements, compounds and mixtures.
Answers
Answer:
An element is a simple atom but a compound has two or more atoms combined together whereas a mixture contains both the elements and components
Explanation:
Basic classification is explained above .
for more information regarding elements compounds and mixture,
https://brainly.in/question/4395449
Balanced equation
Fe + HCI --> FeCl2 + H2
Answers
Answer:
the balanced form is: Fe + 2HCl --> FeCl2 + H2
A stream containing 30% ethanol (molecular weight 46) and the rest is water (molecular weight 18). The mass flow rate of the stream is 100 kg/h. The molar flow rate of water is:
Answers
Based on the mass flow rate of the mixture, the molar flow rate of water is 3.89 mol/h
What is the molar flow rate of a fluid?The molar flow rate of a material refers to the number of moles of a material that passes through a given point of reference within a unit time interval.
The molar flow rate = moles / time
From the data provided:
The mass flow rate of the mixture is 100 kg/h
Percent mass of water = 70%
Hence mass flow rate of water = 70 g/h
molar mass of water = 18 g/mol
Molar flow rate of water = 70 g/h ÷ 18g/mol
Molar flow rate of water = 3.89 mol/h
Learn more about molar flow rate at: https://brainly.com/question/26061120
#SPJ1
Match the term with the definition. (4 points)
1.Solid
2.Liquid
3. Gas
4. Plasma
a. assumes the shape of the part of the container from the bottom up
b. charged particles that do not have a definite shape or volume
c. has a fixed volume and shape
d. takes the shape and volume of an entire container
Answers
Answer:
Liquid - A.
Solid - C.
Gas - D.
Plasma - B.
Explanation:
A liquid sinks to the bottom of a container, a solid is solid and has a fixed shape and density, a gas takes up an entire object (air for example), and plasma is left with B.
Hope this helps! Let me know.
I need help with this chemistry question.
If the percent by mass of carbon in sucrose is 42.2%, then how many grams of carbon are in a 30.0 g sample of sucrose?
_______________ g
Use the correct sigfigs in your answer or the computer will mark it incorrect
Answers
Answer:
12.66g
Explanation:
42.2% of 30.0g
=42.2/100 * 30.0
=0.422*30.0
12.66g
select all of the following physical properties that involve chemical reactions. a. dynamite explodes
b. meat rots if it is not refrigerated
c. gasoline burns
d. ice floats on top of liquid water
e. a silver platter tarnishes
Answers
Dynamite explodes, meat rots if it is not refrigerated, gasoline burns, a silver platter tarnishes
Physical properties refer to characteristics of matter that can be observed or measured without changing the substance's chemical composition. They include properties like color, density, melting point, boiling point, etc.
Chemical reactions, on the other hand, involve the transformation of substances into different substances with different chemical properties.
Dynamite explodes: This is a chemical reaction where the explosive compounds in dynamite rapidly decompose, releasing gases and causing an explosion.
Meat rots if it is not refrigerated: Rotting of meat involves the chemical decomposition of proteins and other organic molecules by microorganisms, leading to the production of foul-smelling compounds.
Gasoline burns: Burning of gasoline is a chemical reaction known as combustion. It involves the reaction of gasoline (hydrocarbon) with oxygen, resulting in the release of energy, heat, and the formation of carbon dioxide and water.
Ice floats on top of liquid water: This is a physical property related to the density of water. Ice is less dense than liquid water, which allows it to float on top.
A silver platter tarnishes: Tarnishing of silver involves a chemical reaction between silver and sulfur compounds in the air, resulting in the formation of a dark layer (silver sulfide) on the surface of the silver object.
The physical properties that involve chemical reactions among the given options are dynamite exploding, meat rotting if not refrigerated, gasoline burning, and a silver platter tarnishing.
To learn more about Gasoline , visit
brainly.com/question/2155980
#SPJ11
What is the molarity of a solution containing 9.0 moles of solute in 462 mL of solution
Answers
Answer:
\(\boxed {\boxed {\sf 19 \ M}}\)
Explanation:
Molarity is a measure of concentration in moles per liter, so the formula is:
\(molarity= \frac{moles \ of \ solute}{liters \ of \ solution}}\)
This solution has 9.0 moles of solute and 462 milliliters of solution. We must convert milliliters to liters. Remember that 1 liter contains 1000 milliliters.
Create a ratio.
\(\frac{ 1 \ L}{ 1000 \ mL}\)Multiply by the value we are converting: 462 milliliters
\(462 \ mL *\frac{ 1 \ L}{ 1000 \ mL}\)\(462 *\frac{ 1 \ L}{ 1000}\)\(0.462 \ L\)
Now we know both values and we can solve for the molarity.
moles of solute= 9.0 moles liters of solution = 0.462 L\(molarity= \frac{9.0 \ mol}{ 0.462 \ L}\)
Divide.
\(molarity= 19.4805195 \ mol/L\)
The original measurements of moles and milliliters have 2 and 3 significant figures respectively. We have to round our answer to the least number of sig figs, which is 2 in this case.
For the number we found, that is the ones place. The 4 in the tenths place (19.4805195) tells us to leave the 9 in the ones place.
\(molarity= 19 \ mol/L\)
1 mole per liter is equal to 1 molar or M, so our answer is equal to 19 M.
\(molarity \approx 19 \ M\)
The molarity of the solution is approximately 19 M.
What is the frequency of a photon with an energy of 3.38 x 10-19 J?
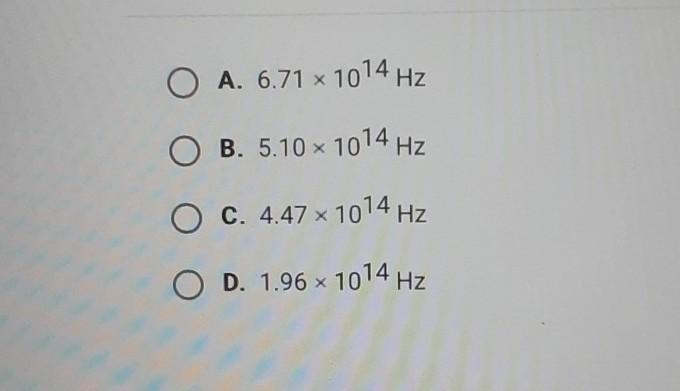
Answers
\(\huge\boxed{5.1x\(10^{14}\)Hz}\)
_____________________________________DATA:E = \(3.38 x 10^{-19}\)
h = \(6.625x10^{-34}\)
f = ?
_____________________________________SOLUTION:Energy of Photon is given by,
E = hf
Rearrange the equation,
f = \(\frac{E}{h}\)
where,
E = energy
h = planck's constant
f = frequency
------------------------------------------------------------------------------------------------------------
Substitute the variable in the equation,
f = \(\frac{3.38x10^{-19}}{6.62x10^{-34}}\)
Simplify the equation,
f = 5.10 x 10^14 Hz
_____________________________________Best Regards,'Borz'
Answer:
b. 5.10 x 10^14 Hz
Explanation:
7. A coal contains 0.15 ppmm of elemental mercury, Hg. For simplicity and conservatism, assume that all the mercury is emitted as elemental mercury. If a power plant burns this coal at a rate of 90 tons/h and operates 85% the year, estimate the annual emissions of mercury [kg/y].
Answers
The annual emissions of mercury from burning the coal at a rate of 90 tons/h, assuming 85% annual operation, can be estimated to be approximately 0.85 kg/y.
To calculate the annual emissions of mercury, we need to consider the concentration of elemental mercury in the coal and the rate at which the coal is burned.
Given that the coal contains 0.15 ppmm (parts per million by mass) of elemental mercury, we can convert this concentration to a fraction by dividing it by 1 million: 0.15/1,000,000.
To find the mass of mercury emitted per hour, we multiply the mercury concentration by the mass of coal burned per hour:
Mass of mercury emitted per hour = (0.15/1,000,000) * 90 tons
Since the power plant operates 85% of the year, the annual emissions can be calculated by multiplying the mass of mercury emitted per hour by the number of hours in a year (365 days * 24 hours) and then multiplying by the operational percentage (85%):
Annual emissions of mercury = (0.15/1,000,000) * 90 tons * (365 days * 24 hours) * 0.85
After converting tons to kilograms (1 ton = 1000 kg), we can simplify the calculation:
Annual emissions of mercury ≈ (0.15/1,000,000) * 90 * 1000 * (365 * 24) * 0.85 kg
Evaluating this expression gives us the estimated annual emissions of mercury from the coal combustion.
To know more about mercury , click;
brainly.com/question/2279846
#SPJ11
What is the pH of 400. mL of solution containing 0.0112 gram of HNO3?
a. 4.15
b. 3.35
c. 10.65
d. 3.75
e. 2.95
Answers
To determine the pH of the solution containing 0.0112 grams of HNO3, we need to first calculate the molarity of the acid.
The molar mass of HNO3 is 63.01 g/mol (1+14.01+48.00), so we can calculate the number of moles of HNO3 present in the solution as follows:
0.0112 g HNO3 x (1 mol HNO3/63.01 g HNO3) = 1.78 x 10^-4 mol HNO3
The volume of the solution is given as 400 mL, which is equivalent to 0.4 L. Therefore, the molarity of the HNO3 solution is:
Molarity = moles of solute/volume of solution in liters
Molarity = 1.78 x 10^-4 mol HNO3 / 0.4 L = 4.45 x 10^-4 M
To determine the pH of the solution, we need to use the equation:
pH = -log[H+]
where [H+] is the concentration of hydrogen ions in the solution. Since HNO3 is a strong acid, it will completely dissociate in water to form H+ and NO3- ions. Therefore, the concentration of hydrogen ions in the solution is equal to the molarity of the HNO3 solution:
[H+] = 4.45 x 10^-4 M
Substituting this value into the equation for pH, we get:
pH = -log(4.45 x 10^-4) = 3.35
Therefore, the pH of the solution containing 0.0112 grams of HNO3 is 3.35. The answer is (b).
Learn more about molarity here:
https://brainly.com/question/8732513
#SPJ11
In a converter, 9.33 kg of SO3 is fed and allowed to come into contact with a certain amount of 91.34% H2SO4 to produce 4.71% oleum. How much oleum was produced in kg? Use the following molecular weights: 80 kg/kmol SO3, 98 kg/kmol H2SO4.
Answers
To solve this problem, we need to determine the amount of oleum produced when 9.33 kg of SO3 reacts with a certain amount of 91.34% H2SO4 to produce 4.71% oleum.
Let's first calculate the mass of H2SO4 present in the initial solution. Since the solution is 91.34% H2SO4, we have:
Mass of H2SO4 = 91.34% * 9.33 kg = 8.51 kg
Next, we can calculate the mass of oleum produced. Since the oleum concentration is 4.71%, we have:
Mass of Oleum = 4.71% * 9.33 kg = 0.439 kg
Therefore, approximately 0.439 kg of oleum was produced.
to learn more about specific volume, visit:
brainly.com/question/10831664
#SPJ11
How many atoms are there in 7.95 moles of carbon dioxide?
Answers
Answer:3
Explanation:
name the gas which is formed when coal is heated in the absense of air
Answers
Answer:
Coke
Explanation:
A solid fuel formed by heating coal in the absence of air is coke. Coke is black colored, tough porous substance. It is pure carbon.
click on the picture of the flowers until their colors correspond to the punnett square above. HELP PLZ!

Answers
Answer:
You will need to have a red flower in the top left, a pink flower in the top right and bottom left, and a white flower in the bottom right.
Explanation:
This flower appears to express color through codominance, which means that both genes express themselves. "R" is the dominant gene for red, and "r" is the recessive gene for white. When a flower has RR genotype, the phenotype will be a red flower. When a flower has an Rr genotype, the red and white genes will both be expressed and the phenotype will be pink. When a flower has an rr genotype, it lacks the red gene and will therefore be only white.
Detects radio waves from objects in space:
a) Radio Telescopes
b) Reflection Telescope
c) Compound telescope
d) Refraction telescope
Answers
Answer:
The correct answer is A
Explanation:
A radio telescope is used to detect, collect and focus radio waves from distant objects in the sky or space. While a compound telescope, by design, can both refract and reflect waves. A reflection telescope uses curved mirrors to reflect light to form an image. A refraction telescope forms an image using a lens as its objective.
From the above definitions, it can be deduced that option A is the correct answer.
determine the electron geometry, molecular geometry, and idealized bond angles for each molecule. in which cases do you expect deviations from the idealized bond angle? a. pf3 b. sbr2 c. chcl3 d. cs2
Answers
Electron geometry, molecular geometry, and idealized bond angles are : a. PF₃ - tetrahedral, trigonal pyramidal, 109.5 degrees, with deviations expected due to lone pairs on the central atom, b. SBr₂ - tetrahedral, bent or V-shaped, 109.5 degrees, with deviations expected due to lone pairs on the central atom, c. CHCl₃ - tetrahedral, tetrahedral, 109.5 degrees, with deviations expected due to lone pairs on the central atom, d. CS₂ - linear, linear, 180 degrees, with no deviations expected.
PF₃ has a central phosphorus atom surrounded by three fluorine atoms. The electron geometry of PF₃ is tetrahedral as there are four electron groups around the central atom. The molecular geometry of PF₃ is trigonal pyramidal, as the three fluorine atoms are not symmetrically placed around the central atom, giving it a pyramidal shape. The idealized bond angle in PF₃ is 109.5 degrees. However, we can expect deviations from this angle due to lone pairs of electrons on the central atom, which can repel the bonding pairs and slightly decrease the bond angle.
Moving on to molecule b, which is SBr₂.
SBr₂ has a central sulfur atom surrounded by two bromine atoms. The electron geometry of SBr₂ is also tetrahedral as there are four electron groups around the central atom. However, the molecular geometry of SBr₂ is bent or V-shaped, as the two bromine atoms are not symmetrically placed around the central atom, giving it a bent shape. The idealized bond angle in SBr₂ is 109.5 degrees, but we can expect deviations from this angle due to the lone pairs of electrons on the central atom, which can slightly decrease the bond angle.
Moving on to molecule c, which is CHCl₃.
CHCl₃ has a central carbon atom surrounded by three hydrogen atoms and one chlorine atom. The electron geometry of CHCl₃ is tetrahedral, as there are four electron groups around the central atom. The molecular geometry of CHCl₃ is also tetrahedral, as the three hydrogen atoms and one chlorine atom are symmetrically placed around the central atom, giving it a tetrahedral shape. The idealized bond angle in CHCl₃ is 109.5 degrees, but we can expect deviations from this angle due to the lone pairs of electrons on the central atom, which can slightly decrease the bond angle.
Finally, molecule d is CS₂.
CS₂ has a central carbon atom surrounded by two sulfur atoms. The electron geometry of CS₂ is linear, as there are two electron groups around the central atom. The molecular geometry of CS₂ is also linear, as the two sulfur atoms are placed symmetrically around the central atom, giving it a linear shape. The idealized bond angle in CS₂ is 180 degrees, and we do not expect any deviations from this angle as there are no lone pairs of electrons on the central atom.
In summary, the electron geometry, molecular geometry, and idealized bond angles for each molecule are:
a. PF₃ - tetrahedral, trigonal pyramidal, 109.5 degrees, with deviations expected due to lone pairs on the central atom
b. SBr₂ - tetrahedral, bent or V-shaped, 109.5 degrees, with deviations expected due to lone pairs on the central atom
c. CHCl₃ - tetrahedral, tetrahedral, 109.5 degrees, with deviations expected due to lone pairs on the central atom
d. CS₂ - linear, linear, 180 degrees, with no deviations expected.
To know more about electron geometry, refer
https://brainly.com/question/7283835
#SPJ11
Convert 1. 709 x 10-5 cm3 to μm3 and express your answer with the correct number of significant figures
Answers
To convert 1.709 x 10^(-5) cm³ to μm³, we need to know the conversion factor between cm³ and μm³.
1 cm is equal to 10,000 μm since 1 cm = 10 mm and 1 mm = 1000 μm. Therefore, 1 cm³ is equal to (10,000 μm)³.
Calculating the conversion factor:
(10,000 μm)³ = 1,000,000,000,000 μm³
Now, we can convert the given value:
1.709 x 10^(-5) cm³ * 1,000,000,000,000 μm³ / 1 cm³ = 1.709 x 10^(-5) x 1,000,000,000,000 μm³ / 1 = 1.709 x 10^7 μm³
Since the given value has 4 significant figures (1.709), we need to express the final answer with the same number of significant figures. Therefore, the converted value of 1.709 x 10^(-5) cm³ to μm³, with the correct number of significant figures, is approximately 1.709 x 10^7 μm³.
Learn more about factor between cm³ and μm³ here
https://brainly.com/question/13980344
#SPJ11
Which types of reactions would result in a color change?
Answers
Answer:
reversible redox reaction
Explanation:
Calculate the activation energy in kJ/mol for a reaction that takes place at 298K with a rate constant of 6.87 x 10-2 and the same reaction that takes place at 325 K with a rate constant of 1.5 x 10-
Answers
The calculated activation energy is Ea/ ⁸°³¹⁴ₓ²⁹⁸ = 0.220
Every molecule has a specific quantity of energy at a minimum. However, if the molecules collide with enough kinetic energy and in the right collision orientation to overcome the minimum energy barrier, a reaction will take place. The activation energy, Ea, is the minimal amount of energy needed for a chemical reaction to take place.
The Arrhenius equation, k=Ae-Ea/RT, can be used to calculate the rate constant.
k being the rate constant
Frequency factor = A
R = Gas constant, where Ea = Activation energy
T = Absolute Temperature, where
1.5x10⁻² =6.87x 10⁻²s⁻¹x e⁻ (Ea / 8.314 J/(mol K) x 298 K)
0.220 = Ea / 8.314 x 298
Ea/ ⁸°³¹⁴ₓ²⁹⁸ = 0.220
Learn more about activation energy here-
https://brainly.com/question/11334504
#SPJ4
What is the total number of grams of HI in 0.500 liter of
1.00 M HI?
64.0 g
128 g
1.00 g
0.500 g
Answers
Answer:
The amount of HI is "64 grams".
Explanation:
The given values are:
Volume,
= 0.500 L
Molarity,
= 1.00 H
Molar mass of HI,
= 128
Now,
The moles of HI will be:
= \(Molarity\times Volume\)
On substituting the values, we get
= \(1.00\times 0.500\)
= \(0.5 \ mol\)
hence,
The amount of HI will be:
= \(Moles\times Molar \ mass\)
= \(0.5\times 128\)
= \(64 \ grams\)
Protein A has a binding site for ligand X with a dissociation constant, Kd, of 3.0 x 10-7 M. Protein B has a binding site for ligand X with a K of 4.0 x 10 M. Calculate the K, for each protein.
Answers
The breakdown constant, Kd, of the binding site onto protein A for ligand X is 3.0 x 10-7 M. A ligand X binding site on protein B does indeed have a K value 4.0 x 10 M. K has a ratio of 0.133.
Describe protein.Protein, which is located in practically every cell, muscle, other body part, encompassing muscle, osteoporosis, skin, and hair, makes up the human body. It helps to produce enzymes, which power countless phase changes, and haemoglobin, which carries oxygen in the blood.
Protein A kd = 3.0 x 10⁻⁷ M
Protein B kd = 4.0 x 10⁻⁸ M
ka = ?
Protein A ka = 1/kd
= 1/ 3.0 x 10⁻⁷
ka = 0.33 * 10⁷m⁻
Protein B ka = 1/kd
= 1/ 4* 10⁻⁸
= 0.25 * 10⁸
ka = 2.5 * 10⁷ m⁻
ratio of k = 4.0 x 10⁻⁸/3.0 x 10⁻⁷
= 0.133
To know more about protein visit:
https://brainly.com/question/29776206
#SPJ4
Can you help plzzz thank you
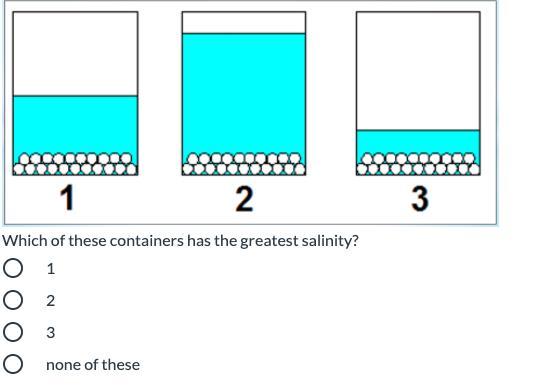
Answers
Answer:
It would be Container 3
Explanation:
Each of the containers has the same amount of salt. Salinity refers to salt level. Since the question is asking for the container with the greatest salinity, you are looking for the container with the least water (because it will be the saltiest out of all of them). Container 3 has the least water.
Hope this helps :)
3 has less water so its saltier