Answers
Answer:
40 grams of KCI are required to make 500 ml of 8% solution.
Related Questions
I place 3 moles of N2 and 4 moles of O2 in a 35 L container at ta temperature of 25 degree Celsius, what will the pressure (in atm) of the resulting mixture of gases be?
Answers
Answer:
The pressure of the resulting mixture of gases will be 4.89 atm.
Explanation:
An ideal gas is a theoretical gas that is considered to be composed of randomly moving point particles that do not interact with each other. Gases in general are ideal when they are at high temperatures and low pressures.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P*V = n*R*T
where P is the gas pressure, V is the volume that occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas. The universal constant of ideal gases R has the same value for all gaseous substances. The numerical value of R will depend on the units in which the other properties are worked.
In this case:
P= ?V= 35 Ln= 3 moles of N₂ + 4 moles of O₂= 7 molesR= 0.082 \(\frac{atm*L}{mol*K}\)T= 25 C= 298 K (being 0 C= 273 K)Replacing:
P* 35 L= 7 moles* 0.082 \(\frac{atm*L}{mol*K}\) * 298 K
Solving:
\(P=\frac{ 7 moles* 0.082 \frac{atm*L}{mol*K} * 298 K}{35 L}\)
P= 4.89 atm
The pressure of the resulting mixture of gases will be 4.89 atm.
The pressure (in atm) of the resulting mixture of the gases is 4.89 atm
From the question given above, the following data were obtained:
Mole of N₂ = 3 moles
Mole of O₂ = 4 moles
Total mole = 3 + 4 = 7 moles
Volume (V) = 35 L
Temperature (T) = 25 °C = 25 + 273 = 298 K
Gas constant (R) = 0.0821 atm.L/Kmol
Pressure of mixture (P) =?The pressure of the resulting mixture of the gases can be obtained by using the ideal gas equation as illustrated below:
PV = nRT
P × 35 = 7 × 0.0821 × 298
P × 35 = 171.2606
Divide both side by 35
P = 171.2606 / 35
P = 4.89 atmTherefore, the pressure of the resulting mixture of the gases 4.89 atm
Learn more: https://brainly.com/question/21912477
If you have 9.4 x 10^24 molecules of gold how many grams of gold do you have?
Answers
3,075.61 grams
Explanations:According to the Avogadro's constant;
\(1\text{mole}=6.02\times10^{23}molecules\)Convert the molecules of Gold to mole using the conversion factor:
\(\begin{gathered} \text{moles of Au=}\frac{9.4\times10^{24}}{6.02\times10^{23}} \\ \text{moles of Au=}1.56\times10^{24-23} \\ \text{moles of Au=}1.56\times10 \\ \text{moles of Au=}15.6\text{moles} \end{gathered}\)Determine the mass of Gold
\(\begin{gathered} \text{Mass}=\text{mole}\times\text{molar mass} \\ \text{Mass}=15.6\cancel{\text{moles}}\times\frac{196.97g}{\cancel{\text{mol}}} \\ \text{Mass}=3,075.61\text{grams} \end{gathered}\)Hence the mass of Gold is 3,075.61 grams
How many formula units make up 20.6 g of magnesium chloride (MgCl2)?
Answers
Answer:The formula of magnesium chloride is MgCl2 . The molar mass of MgCl2 is (24.30 + 2 × 35.45) g/mol=95.20 g/mol .
Explanation:
write a balanced chemical equation for the decomposition of asprin
Answers
The balanced chemical equation for the decomposition of aspirin (acetylsalicylic acid) is:
\(2C_{9}H_{8}O_{4} (aspirin) → 2C_{7}H_{6}O_{3} (salicylic acid) + 2CO_{2} (Carbon dioxide) + H_{2}O (water)\)
In this reaction, the aspirin molecule breaks down into salicylic acid, carbon dioxide, and water. The reaction is typically catalyzed by heat or exposure to acidic or basic conditions.
Aspirin, or acetylsalicylic acid, contains ester functional groups that can undergo hydrolysis. Under suitable conditions, the ester bond in aspirin is cleaved, leading to the formation of salicylic acid, which is the primary decomposition product. Additionally, carbon dioxide and water are released as byproducts of the reaction.
The balanced equation shows that for every two molecules of aspirin, two molecules of salicylic acid, two molecules of carbon dioxide, and one molecule of water are formed. Understanding the decomposition of aspirin is important in pharmaceutical and chemical industries to ensure the stability and shelf-life of the compound, as well as to study its breakdown products and potential side reactions.
Know more about aspirin here:
https://brainly.com/question/13533428
#SPJ8
What must happen for a nuclear reactor to make electricity
Answers
Answer:
They contain and control nuclear chain reactions that produce heat through a physical process called fission. That heat is used to make steam that spins a turbine to create electricity
Explanation:
A dam constructed to produce tidal power does so by _____. reducing the range between high tide and low tide harnessing water flow to drive turbines and electric generators protecting a coastal area from large ocean waves preventing saltwater from moving from the ocean into a bay
Answers
A dam constructed to produce tidal power does so by harnessing water flow to drive turbines and electric generators .
What purposes does tidal energy serve?Tidal energy was employed in grain mills to crush grains mechanically, just like wind energy was. grain crushing Here, the tidal energy generated by the turbines was used. Hydroelectric dams, which serve as significant energy storage, also employ tidal energy to store energy.
Tidal power can harm marine life because tidal turbines' whirling blades can cause marine organisms to perish. Fish habitations in tidal power settings may be impacted by noise from the turbines' rotation. Tidal energy can also affect how sediment and water are processed.
Therefore, option B is correct.
Learn more about tidal power at:
https://brainly.com/question/10578402
#SPJ1
How many inches are in 4.5 miles?
Answers
63360 x 4.5 = 285,120 inches
so there is 285,120 inches in 4.5 miles
How many molecules are shown in the chemical formula pictured? H3PO4
A) 1
B) 3
C) 4
D) 7
Answers
Answer
A(1)
Explanation:
If you don't see a coefficient, which is the big number in front of the formula, then it's only one molecule.
Example of coefficient
4H3PO4
.5 moles of lithium chloride are dissolved in .05 liters of water. What is the molarity of the solution?
Answers
0.5 moles of lithium chloride are dissolved in .05 liters of water. 10M is the molarity of the solution.
The total amount of moles of solute found within a specific number of litres of the solution, or moles per litre of a solution, is known as molar concentration or molarity. Please explain the difference amongst the terms "solute" and "solvent" before we continue.
'Solution' for making it simpler to comprehend the topics that will follow. Solutes are simply substances that exist in solutions because a solution is defined as a homogenous mixture that comprises one or more solutes.
Molarity = moles/volume of solution in liter
= 0.5/ .05
= 10M
To know more about molarity, here:
https://brainly.com/question/8732513
#SPJ1
Plz help ASAP
Plz and ty

Answers
Answer:
It's circulatory system
Hope it's help ^_^
Answer: circilutory
Explanation:
plse help dont understand

Answers
The origin for the word Cent is French and another example is centimeter.
The origin for Bi is Latin and another example is bilingual.
The origin for the word Ism is greek and another example is racism.
The origin for the word graph is greek and another word is biography.
The origin for the word form is latin and another example is formula.
the origin for the word cycl is greek and another example is bicycle.
The magnitude of one Kelvin, one Celsius degree, and one degree on the absolute temperature scale is the same. true or false . please explain it .....
Answers
Answer:
false
Explanation:
I don't think centigrade ranges from 0 to 100 and kelvin 237 and 373 absolute -273°c and 0K
Answer:
false............................
Magnesium hydroxide reacts with chlorine to form magnesium chloride,
magnesium chlorate and water. How many grams of magnesium hydroxide is
needed to yield 8.00 moles of magnesium chlorate?
77.8 g Mg(OH)2
9178.1 g Mg(OH)2
2799.6 g Mg(OH)2
.823 g Mg(OH)2
How many grams of sodium sulfato pro
Answers
The grams of magnesium hydroxide needed to yield 8.00 moles of magnesium chlorate is approximately 466.64 g. None of the options provided match the calculated value of 466.64 g.
To determine the grams of magnesium hydroxide (Mg(OH)2) needed to yield 8.00 moles of magnesium chlorate (Mg(ClO3)2), we need to consider the balanced chemical equation for the reaction between magnesium hydroxide and chlorine.
The balanced equation is as follows:
2 Mg(OH)2 + 6 Cl2 → 2 Mg(ClO3)2 + 2 H2O
From the balanced equation, we can see that 2 moles of Mg(OH)2 react with 6 moles of Cl2 to produce 2 moles of Mg(ClO3)2.
Therefore, the stoichiometric ratio is 2 moles of Mg(OH)2 : 2 moles of Mg(ClO3)2.
To calculate the grams of Mg(OH)2 needed, we can use the stoichiometric ratio and the given moles of Mg(ClO3)2.
Given:
Moles of Mg(ClO3)2 = 8.00 moles
Using the stoichiometric ratio, we have:
8.00 moles Mg(ClO3)2 × (2 moles Mg(OH)2 / 2 moles Mg(ClO3)2) = 8.00 moles Mg(OH)2
To convert moles to grams, we need to multiply by the molar mass of Mg(OH)2.
The molar mass of Mg(OH)2 = (24.31 g/mol) + (2 * 16.00 g/mol) = 58.33 g/mol
Grams of Mg(OH)2 = 8.00 moles Mg(OH)2 × 58.33 g/mol = 466.64 g
Therefore, the grams of magnesium hydroxide needed to yield 8.00 moles of magnesium chlorate is approximately 466.64 g.
For more such questions on magnesium chlorate
https://brainly.com/question/12358640
#SPJ11
Diamond and graphite are two crystalline forms of carbon. At 1 atm and 25°C, diamond changes to graphite so slowly that the enthalpy change of the process must be obtained indirectly. Determine ΔHrxn for
C(diamond) → C(graphite)
with equations from the following list:
(1) C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ
(2) 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ
(3) C(graphite) + O2(g) → CO2(g) ΔH = −393.5 kJ
(4) 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJ
Answers
The enthalpy change of the reaction C(diamond) → C(graphite) is -2.9 kJ.
The given information is ΔHrxn for the reaction C(diamond) → C(graphite) can be calculated with the given equations:Equations: C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ C(graphite) + O2(g) → CO2(g) ΔH = −393.5 kJ 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJThe required reaction can be obtained by adding the equations (1) and (4), as follows:C(diamond) + O2(g) + 2CO(g) → C(graphite) + 3CO2(g)Addition of the two equations (1) and (4) results in a reaction whose products are C(graphite) and CO2.
To get the final equation that involves only the required reactants and products, the equation (2) should be added, which consumes CO2 and produces O2, as shown below:C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ [eq. (1)] 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJ [eq. (4)] 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ [eq. (2)] C(diamond) + O2(g) + 2CO(g) → C(graphite) + 3CO2(g) ΔHrxn=ΣΔHf(products)−ΣΔHf(reactants) ΔHrxn=[(3 mol CO2)(-393.5 kJ/mol) + (1 mol C(graphite))(0 kJ/mol)] − [(1 mol C(diamond))(0 kJ/mol) + (1 mol O2)(0 kJ/mol) + (2 mol CO(g))(−172.5 kJ/mol)] − [(2 mol CO2)(566.0 kJ/mol)] ΔHrxn=−2.9 kJ.
for such more questions on reaction
https://brainly.com/question/11231920
#SPJ8
What happens to electrolytes when dissolved in water?They break into protonsThey break into ionsThey break into isotopesThey break into electrons
Answers
Explanation:
By definition the substances that give ions when dissolved in water are called electrolytes. Electrolytes dissociate into cations (positively charged ions) and anions (negatively charged ions) when dissolved in water.
Answer: They break into ions
PLEASE HELP
We wish to determine the moles of solid AgCl formed when 50.0 ml of 0.250 M AgNO3 reacts with excess MgCl2 according to the equation below.
2AgNO3(aq) + MgCl2(aq) 2Ag Cl(s) + Mg (NO3)2(aq)
In the previous step you determined 0.0125 mol AgNO3 react. How many moles of AgCl form during the reaction?
Answers
The number of moles of AgCl formed during the reaction is 0.0125 mol.
Given the reaction:2AgNO3(aq) + MgCl2(aq) → 2Ag Cl(s) + Mg (NO3)2(aq)We are supposed to determine the moles of solid AgCl formed when 50.0 ml of 0.250 M AgNO3 reacts with excess MgCl2 and in the previous step, we found that 0.0125 mol of AgNO3 reacts.
We can use the stoichiometry method to find the moles of AgCl formed.
To do so, we will have to balance the given chemical equation and find out the number of moles of AgCl formed from the given reactants.
The balanced chemical equation is:2AgNO3(aq) + MgCl2(aq) → 2Ag Cl(s) + Mg (NO3)2(aq)From the equation, we can say that 2 moles of AgCl form from 2 moles of AgNO3 reacted.
In the previous step, we have found the number of moles of AgNO3 reacted, which is 0.0125 mol.
As per the balanced chemical equation, 2 moles of AgCl form from 2 moles of AgNO3 reacted.
Therefore, the number of moles of AgCl formed = (0.0125 mol AgNO3 reacted × 2 moles AgCl / 2 moles AgNO3) = 0.0125 mol AgCl.
The number of moles of AgCl formed during the reaction is 0.0125 mol.
For more questions on AgCl
https://brainly.com/question/15393967
#SPJ8
As the climate warms, ice and
snow melt. This makes the
climate hotter, which then
melts more snow and ice. This
is an example of
A. positive feedback
B. negative feedback
C. neutrality
Answers
The scenario in which as the climate warms, ice and snow melt making the climate hotter, which then melts more snow and ice is an example of positive feedback.
The correct option is A.
What is positive feedback?Positive feedback refers to a process in which an initial change or disturbance in a system leads to an amplification or reinforcement of that change.
Considering the scenario of climate change, the example provided earlier is a demonstration of positive feedback.
As the climate warms, ice and snow melt, reducing the reflective surface and exposing darker surfaces like land or water. These darker surfaces absorb more sunlight, which leads to further warming and more melting of ice and snow. This cycle continues, causing a self-reinforcing effect that amplifies the initial warming.
Learn more about positive feedback at: https://brainly.com/question/28271726
#SPJ1
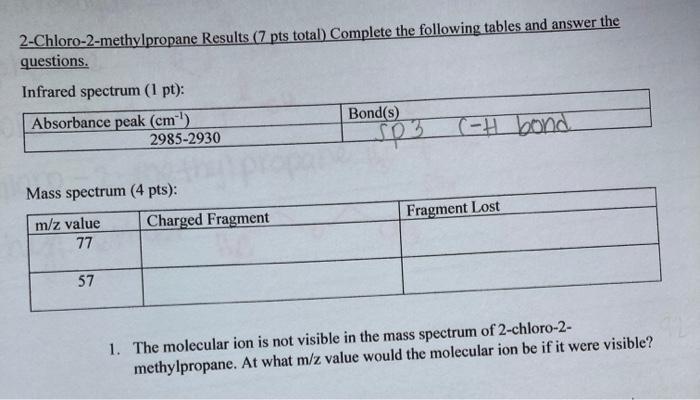
The molecular ion is not visible in the mass spectrum of 2-chloro-2- methylpropane. At what m/z value would the molecular ion be if it were visible? What evidence is there in the mass spectrum that suggests that the peak at m/z= 77 contains a chlorine atom?
Answers
Answer: hello the complete question is attached below
Visibility of molecular ion = m/z value of 77
Explanation:
For The molecular ion to be visible, it has to be at an m/z value of 77 and this is because molecular ions will have an m/z ratio = molecular mass of given molecule in most cases but not always in all cases.
And the visibility is possible after the removal of CH₃ ion.
ii) Evidence in the mass spectrum that suggests peak at m/z = 77
attached below

A student obtains a beaker containing a solution that is determined to have a hydrogen ion
concentration (H+) of 6.7x10-14. Based on this information, please answer the following
questions:
a) What is the pH of the solution?
b) is the solution acidic, neutral or basic?
Answers
Answer:
pH = 13.1
Basic
Explanation:
Step 1: Given data
Hydrogen ion concentration ([H⁺]): 6.7 × 10⁻¹⁴ M
Step 2: Calculate the pH of the solution
We will use the definition of pH.
pH = -log [H⁺]
pH = -log 6.7 × 10⁻¹⁴ = 13.1
When pH < 7, the solution is acid.When pH = 7, the solution is neutral.When pH > 7, the solution is basic.Since pH > 7, the solution is basic.
Which of these does not accurated descibe the wright brothers first airplane
Answers
Answer:
it had wings that flapped similat to birds wings
Calculate standard cell potential of an electrochemical cell powered by these half-reactions. (Write values to two decimal places. If a value is less than 1, be sure to write a 0 before the decimal.)
Pb4+ + 2e− → Pb2+
Co3+ + e− → Co2+
E°cell = V
Is the reaction spontaneous
Answers
The standard cell potential is found as +1.95 V and is a spontaneous reaction.
What is standard cell potential ?The standard cell potential (E°cell) of an electrochemical cell is given by the difference between the standard reduction potentials of the two half-cells involved.
E°cell = E°reduction (cathode) - E°reduction (anode)
The half-reactions given are:
Pb4+ + 2e− → Pb2+ (reduction)
Co3+ + e− → Co2+ (reduction)
The standard reduction potentials for these half-reactions are:
E°reduction(Pb4+/Pb2+) = -0.13 V
E°reduction(Co3+/Co2+) = +1.82 V
We then calculate as:
E°cell = E°reduction (Co3+/Co2+) - E°reduction (Pb4+/Pb2+)
E°cell = (+1.82 V) - (-0.13 V)
E°cell = +1.95 V
Learn more about standard cell potential at: https://brainly.com/question/29653954
#SPJ1
3. Do the changes that you observed in the relative solubilities of NaCl agree with their Solubility Curves ? Explain your answer .
Answers
6. As we can see from the graph, if we have only 100 grams of water, at 30°C, we will have 10 grams of KClO3 being dissolved in water, therefore if we have 300 grams of H2O, we will have 30 grams of KClO3 being dissolved at 30°C
7. The least soluble substance will be the one that shows up first in the temperature "line", if you follow the temperature up, the first solute that is listed will be considered to least soluble, therefore the least one will be KClO3
Natural gas is a mixture of many substances, primarily CH4, C2H6, C3H8, and C4H10. Assuming that the total pressure of the gases is 1.48 atm and that their mole ratio is 94 : 4.0 : 1.5 : 0.50, calculate the partial pressure in atmospheres of each gas:
Answers
Answer:
SEE EXPLANATION
Explanation:
We have the mole ratio as; 94 : 4.0 : 1.5 : 0.50
This implies that the total number of natural gas is; 94 + 4.0 + 1.5 + 0.50 = 100 moles
Recall that partial pressure = mole fraction * total pressure
For CH4 = 94/100 * 1.48 atm = 1.39 atm
For C2H6 = 4/100 * 1.48 atm = 0.0592 atm
For C3H8 = 1.5/100 * 1.48 atm = 0.0222 atm
For C4H10 = 0.5/100 * 1.48 atm = 0.0074 atm
How many lbs. of O2 are required to fully convert 1-lb of glucose (C6H12O6) to carbon dioxide and water
Answers
Answer:
The correct answer is : 1.07 lbs
Explanation:
solution:
molar mass glucose (C6H12O6) = 180 g/mol
molar mass of oxygen molecule (O2) = 32 g/mol (as we know molar mass of O = 16 g/mol)
the balanced reaction of conversion of water and oxygen to glucose is:
\(C_{6}H_{12}O_{6} + 6O_{2} \rightarrow 6CO_{2} + 6H_{2}O ...(A)\)
1 mol of C6H12O6 = 6 mol of O2 (from reaction A)
so, 180 g C6H12O6 = 192 g O2
that is, 0.396 lb of C6H12O6 = 0.423 lb of O2 ( 1 g = 0.00220462 lb )
so,
1 lb C6H12O6 =\(\frac{1(lb) \times 0.4233 (lb)}{0.3968 (lb)}\) = 1.07 lb O2
therefore, the correct answer is : 1.07 lbs
I need help with the first one please!

Answers
Explanation:
...........................
.......

6. What do you think Redi's conclusion
was?
a. Living things come from other living
things.
b. Living things are created through
spontaneous generation.
c. He did not have enough data to arrive at a
conclusion.
Answers
Redi's conclusion was He did not have enough data to arrive at conclusion. Option C.
Redi went on to demonstrate that dead maggots or flies would not generate new flies when placed on rotting meat in a sealed jar, whereas live maggots or flies would. This disproved both the existence of some essential components in once-living organisms and the necessity of fresh air to generate life.
Redi's hypothesis developed by Francesco Redi said that living organisms came from other living organisms and not from non-living sources. Redi demonstrated this by covering the meat, as a result, no maggots would emerge. Redi's experiment proved that life maggots from nonlife meat were superstition. propagandizing the ancient Greek spontaneous generation superstitions of 2,300 years earlier.
Learn more about Redi's conclusion here:-https://brainly.com/question/4288726
#SPJ1
How many moles are present in a sample if it consists of 5.61x1022 particles? Report your answer to 3 decimal places. Do not include units.
Answers
Answer:
The mole is defined as a collection of 6.022 × 1023 particles.
The atomic mass given on a periodic table that is given in grams is the mass of
one mole (6.022 × 1023 particles) of that element
Explanation:
How many significant figures are there in the answer for the following problem?
45.3 + 0.7711 + 19 = ?
Answers
Answer:
65,0711__________________
The Ka of hypochlorous acid (HClO) is 3.00*10^-8. What is the pH at 25.0 °C of an aqueous solution that is 0.02M in HClO?
Answers
Answer:
Approximately \(4.6\).
Explanation:
Hypochlorous acid \(\rm HClO\) ionizes partially at room temperature:
\(\rm HClO \rightleftharpoons H^{+} + ClO^{-}\).
The initial concentration of \(\rm HClO\) in this solution is \(0.02\; \rm mol \cdot L^{-1}\).
Construct a \(\verb!RICE!\) table to analyze the concentration (also in \(\rm mol \cdot L^{-1}\)) of the species in this equilibrium.
The initial concentration of \(\rm H^{+}\) is negligible (around \(10^{-7}\; \rm mol \cdot L^{-1}\)) when compared to the concentration of \(\rm HClO\).
Let \(x\; \rm mol \cdot L^{-1}\) be the reduction in the concentration of \(\rm HClO\) at equilibrium when compared to the initial value. Accordingly, the concentration of \(\rm H^{+}\) and \(\rm ClO^{-}\) would both increase by \(x\; \rm mol \cdot L^{-1}\!\). (\(x > 0\) since concentration should be non-negative.)
\(\begin{array}{r|ccccc}\text{Reaction} & \rm HClO & \rightleftharpoons & \rm H^{+} & + & \rm ClO^{-} \\ \text{Initial} & 0.02 & & & &x \\ \text{Change} & -x & & +x & & +x \\ \text{Equilibrium} & 0.02 - x & & x & & x\end{array}\).
Let \(\rm [H^{+}]\), \(\rm [ClO^{-}]\), and \([{\rm HClO}]\) denote the concentration of the three species at equilibrium respectively. Equation for the \(K_\text{a}\) of \(\rm HClO\):
\(\begin{aligned}K_\text{a} &= \frac{\rm [H^{+}] \cdot [ClO^{-}]}{[\rm HClO]}\end{aligned}\).
Using equilibrium concentration values from the \(\verb!RICE!\) table above:
\(\begin{aligned}K_\text{a} &= \frac{\rm [H^{+}] \cdot [ClO^{-}]}{[\rm HClO]} = \frac{x^{2}}{0.02 - x}\end{aligned}\).
\(\begin{aligned}\frac{x^{2}}{0.02 - x} &= 3.00 \times 10^{-8}\end{aligned}\).
Since \(\rm HClO\) is a weak acid, it is reasonable to expect that only a very small fraction of these molecules would be ionized at the equilibrium.
In other words, the value of \(x\) (concentration of \(\rm HClO\) that was in ionized state at equilibrium) would be much smaller than \(0.02\) (initial concentration.)
Hence, it would be reasonable to estimate \((0.02 - x)\) as \(0.02\):
\(\begin{aligned}\frac{x^{2}}{0.02} &\approx \frac{x^{2}}{0.02 - x} = 3.00 \times 10^{-8}\end{aligned}\).
Solve for \(x\) with the simplifying assumption:
\(\begin{aligned}x &\approx \sqrt{0.02 \times {3.00 \times 10^{-8})}}\\ &\approx 2.45 \times 10^{-5}\end{aligned}\).
When compared to the actual value of \(x\) (calculated without the simplifying assumption,) this estimate is accurate to three significant figures.
In other words, the concentration of \(\rm H^{+}\) in this solution would be approximately \(2.45 \times 10^{-5}\; \rm mol \cdot L^{-1}\) at equilibrium.
Hence the \(\text{pH}\):
\(\begin{aligned}\text{pH} &= \log_{10} ([{\rm H^{+}}]) \\ &\approx \log_{10} (2.45 \times 10^{-5}) \\ &\approx 4.6\end{aligned}\).
what is the correct electron configuration of phosphorus (P)?
Answers
Answer: [Ne] 3s² 3p³