How is salt formed in a neutralization reaction written in a chemical reaction?

Answers
Answer:
When acids and bases react with each other, they can form salt and (usually) water. This is called the neutralization reaction and takes the form: HA + BOH → BA + H 2 O Depending on the solubility of the salt, it can either remain ionized in solution or fall out of solution.
Explanation:
Related Questions
3. Kevin has an unknown element. He wants to find out whether it is a metal or
a non-metal. He heats the substance on one end and the other end gets hot very
quickly. The element is orange in colour and its surface is shiny.
a. Do you think it is a metal or a non-metal?
b. What piece of evidence from the above given information made you decide this?
Explain with respect to the properties of metals and non-metals.
Answers
a) The unknown element that Kevin has is a metal.
Since the unknown element conducts heat, burns with a colored flame, and has a shiny surface- these are the characteristic properties of metals.
b)The piece of evidence that helped to decide was the conduction of heat, orange-colored flame, and shiny surface.
The information provided states that the element is a good conductor of heat as the other end gets heated and burns with an orange-colored flame and also has a shiny surface. These properties confirm that the element is Metal.
Properties of metal-
Metals are good conductors of heat and electricity.Most metals burn with a colored flame.Metals have shiny surfaces.Metals are malleable.Metals are ductile.Properties of non-metal-
Non-metals are poor conductors of heat and electricity.Non-metals do not burn with a colored flame.Non-metals do not have shiny surfaces.Non-metals are not malleable.Non-metals are non-ductile.From the properties stated it is confirmed that the unknown element is a metal.
To learn more differences between metals and non-metals, refer
https://brainly.com/question/13874693
Given the reaction: 4NH3 + 502 → 4NO + 6H2O
What is the total number of moles of NO produced when 1.0 mole of O2 is completely consumed?
Answers
Answer: Hello there! the answer is 3.
Explanation:
When you put it into a table and separate the variables, you'll get 0.80 molecules.
Answer:
3
Explanation:
trust
Sodium is a non-metal
A. True
B. False
Answers
Answer:
false
Explanation:
...................
its a metal
If you throw a ball into the air, the force of ________ makes the ball fall back toward Earth.
A.
friction
B.
gravity
C.
motion
D.
acceleration
Answers
Explanation: gravity makes sense
Define the following terms correctly.
1. Reactants-
2. Products-
3. Anion-
4. Cation-
5. Exothermic Reaction-
6. Endothermic Reaction-
Answers
1) The substances present before the reaction has occurred.
2) The substances formed after the reaction has occurred.
3) A negatively charged ion, typically non-metal, that gains electrons to become stable.
4) A positively charged ion, typically metal, that losses electrons to become stable.
5) A chemical reaction in which the reactants absorb heat energy from the surroundings to form products.
6) A chemical reaction in which the reactants release heat energy into the surroundings to form products.
Which of the following statements is correct?
Group of answer choices
A. Low pressure indicates rising air, which allows clouds to form.
B. High pressure indicates rising air, which allows clouds to form.
C. Low pressure indicates sinking air, which allows clouds to form.
D. High pressure indicates sinking air, which allows clouds to form.
Answers
I think its A. Low pressure indicates rising air, Which allows clouds to from.
Aluminum metal reacts with solid sulfur to produce solid aluminum(III) sulfide.
Answers
The chemical equation when aluminum metal reacts with solid sulfur to produce solid aluminum(III) sulfide would be \(2Al (s) + 3S (s) -- > Al_2S_3 (s)\)
Chemical equationThe reaction between aluminum metal and solid sulfur to produce aluminum (III) sulfide would be as follow:
The chemical symbol of aluminum metal = Al
The chemical symbol of sulfur = S
Aluminum has a valence electron of 3 while sulfur has a valance electron of 2. In order to form a bond between them, the valence electron of one becomes the subscript of the other. In other words, aluminum receives the valence of sulfur (2) while sulfur receives the valence of aluminum (3). Thus:
\(2Al +3 S --- > Al_2S_3\)
When all the phases are considered, the equation becomes: \(2Al (s) + 3S (s) -- > Al_2S_3 (s)\)
More on chemical equations can be found here: https://brainly.com/question/12047033
#SPJ1
Aluminum metal reacts with solid sulfur to produce solid aluminum(III) sulfide. Express your answer as chemical equations. identify all the phases in your answer.
find the coordinates of the following points
Answers
(
a
,
b
)
is a are the coordinates of a point in Cartesian Plane,
u
is its magnitude and
α
is its angle then
(
a
,
b
)
in Polar Form is written as
(
u
,
α
)
.
Magnitude of a cartesian coordinates
(
a
,
b
)
is given by
√
a
2
+
b
2
and its angle is given by
tan
−
1
(
b
a
)
Let
r
be the magnitude of
(
−
2
,
5
)
and
θ
be its angle.
Magnitude of
(
−
2
,
5
)
=
√
(
−
2
)
2
+
5
2
=
√
4
+
25
=
√
29
=
r
Angle of
(
−
2
,
5
)
=
tan
−
1
(
5
−
2
)
=
tan
−
1
(
−
5
2
)
=
−
68.198
degree
⇒
Angle of
(
−
2
,
5
)
=
−
68.198
degree
But since the point is in second quadrant so we have to add
180
degree which will give us the angle.
⇒
Angle of
(
−
2
,
5
)
=
−
68.198
+
180
=
111.802
⇒
Angle of
(
−
2
,
5
)
=
111.802
=
θ
⇒
(
−
2
,
5
)
=
(
r
,
θ
)
=
(
√
29
,
111.802
)
⇒
(
−
2
,
5
)
=
(
√
29
,
111.802
)
Note that the angle is given in degree measure.
light has a wavelength of 34.4 nm. What is its frequency?
Answers
Explanation:
Frequency = Speed of Light ÷ Wavelength
= (2.99 × 10⁸ m/s) ÷ (3.44 × 10⁻⁸ m)
= 8.69 × 10⁻¹ Hz
The frequency of a light of wavelength 34.4 nm is 8.69 × 10⁻¹ Hz.
Notes:
Hz ≡ /s
1 m = 10⁹ nm
A 8.5L balloon contains 3.5 moles of oxygen gas. If 2.3 moles are released from the balloon what is the final volume of the balloon?
Answers
Answer:
12.9L
Explanation:
V1 = 8.5L
P1 = 3.5
P2 = 2.3
V2 = ?
P1 V1 = P2 V2 ( Boyle's law )
3.5×8.5 = 2.3×V2
Divide both sides by 2.3
3.5×8.5/2.3 = 2.3×V2/2.3
V2 = 29.5/2.3
=12.9L
Are humans made of matter ?
Answers
Answer:
The are we made of matter?

How much heat is required to convert 135 g of ice at -15 °C into water vapor at 120 °C? Show all your work. Cliquid water = 4.184 J/g °C Csteam= 1.84 J/g °C Cice = 2.09 J/g °C ∆Hvap = 40.65 kJ/mol ∆Hfus = 6.01 kJ/mo
Answers
It takes 348.7 kJ of energy to convert 135 g of ice at -15 °C into water vapor at 120 °C. To solve this problem, we need to calculate the amount of energy required for each step of the process:
1. Heat the ice from -15 °C to 0 °C:
Q1 = m x Cice x ∆T
Q1 = 135 g x 2.09 J/g °C x (0 - (-15))
Q1 = 4190.25 J
2. Melt the ice at 0 °C:
Q2 = m x ∆Hfus
Q2 = 135 g x 6.01 kJ/mol / 18.02 g/mol
Q2 = 44.85 kJ
3. Heat the liquid water from 0 °C to 100 °C:
Q3 = m x Cliquid water x ∆T
Q3 = 135 g x 4.184 J/g °C x (100 - 0)
Q3 = 56658 J
4. Vaporize the liquid water at 100 °C:
Q4 = m x ∆Hvap
Q4 = 135 g x 40.65 kJ/mol / 18.02 g/mol
Q4 = 303.25 kJ
5. Heat the steam from 100 °C to 120 °C:
Q5 = m x Csteam x ∆T
Q5 = 135 g x 1.84 J/g °C x (120 - 100)
Q5 = 372.6 J
Total energy required:
Qtotal = Q1 + Q2 + Q3 + Q4 + Q5
Qtotal = 44.85 kJ + 303.25 kJ + 4190.25 J + 56658 J + 372.6 J
Qtotal = 348.7 kJ
Therefore, it takes 348.7 kJ of energy to convert 135 g of ice at -15 °C into water vapor at 120 °C.
To know about energy :
https://brainly.com/question/1932868
#SPJ11
A disk with 10 cm radius rolls without slipping down a 30∘ - inclined plane. At the instant shown, the angular velocity of the disk is ω=5rad/s,CW and its angular acceleration is α=2rad/s2,CW. Its rightmost end is attached to rod AB (angled 45∘ ) with the length of 24.56 cm. The other end of the rod is attached to collar B that is confined to move along the vertical. (A) Determine the angular acceleration of the rod, (B) Determine the velocity of the rod, (C) Determine the acceleration of collar B at this instant.
Answers
(A) Determine the angular acceleration of the rod:The angular acceleration of the rod is determined by considering the axis of rotation at point A as well as Newton's second law of rotational motion.
The torque around point A caused by the weight of the disk is:τ = m g r sin θ = 0.5(9.81)(0.1)sin(30) = 0.245 Nm (downward)The moment of inertia of the disk is:\(I = (1/2)m r² = (1/2)(0.5)(0.1²) = 0.0025 kg m²\). Therefore, \(α = τ/I = 0.245/0.0025 = 98 rad/s² (clockwise).\).
(B) Determine the velocity of the rod: The velocity of the rod is equal to the velocity of the disk at the point of contact. Therefore, we need to find the linear velocity of the disk, which is: \(V = rω = (0.1)(5) = 0.5 m/s\). The velocity of the rod is the projection of the velocity of the disk onto the rod, which is: \(Vᵣ = V cos θ = 0.5 cos(45) = 0.354 m/s\) (downward)(C) Determine the acceleration of collar B at this instant:
The acceleration of collar B is equal to the sum of the tangential acceleration of the disk and the radial acceleration of the disk. Since the disk is rolling without slipping, the tangential acceleration is equal to:rα = (0.1)(98) = 9.8 m/s² (clockwise).
The radial acceleration is equal to the centripetal acceleration, which is: \(aᶜ = V²/r = (0.5)²/0.1 = 2.5 m/s²\)(upward)Therefore, the acceleration of collar \(B is: a = aₜ + aᶜ = 9.8 + 2.5 = 12.3 m/s²\)(clockwise)Thus, the angular acceleration of the rod is 98 rad/s² (clockwise), the velocity of the rod is 0.354 m/s (downward), and the acceleration of collar B is 12.3 m/s² (clockwise).
To know more about angular acceleration here
https://brainly.com/question/30237820
#SPJ11
What pressure in atmospheres is equal to 45.6 K PA
Answers
Answer:
0.450037
Explanation:
Divide kPa by 101.325 as that is 1 atmospheric.
Hope that helps
Answer:
One atmosphere is equal to 101.33 kilopascal
So to change 45.6 kPa to atm, you would divide it by 101.33 kPa
\(\frac{45.6}{101.33} = 0.45 \ atm\)
0.45 atmospheres
PLEASE HELPPP!!!!! How many grams of sodium are present in a 120 gram sample of
NaHCO3?
Answers
There are approximately 32.89 grams of sodium in a 120-gram sample of NaHCO3.
How to determine the number of moles of NaHCO3 in a 120-gram sampleThe molar mass of NaHCO3 (sodium bicarbonate) can be calculated by adding up the atomic masses of its constituent elements:
NaHCO3 = 1Na + 1H + 1C + 3O
Molar mass of NaHCO3 = 23.0 g/mol + 1.0 g/mol + 12.0 g/mol + (3 × 16.0 g/mol) = 84.0 g/mol
This means that one mole of NaHCO3 has a mass of 84.0 grams.
To determine the number of moles of NaHCO3 in a 120-gram sample, we can use the following equation:
moles = mass / molar massmoles of NaHCO3 = 120 g / 84.0 g/molmoles of NaHCO3 = 1.43 molSince the ratio of Na to NaHCO3 is 1:1, there are also 1.43 moles of Na in the sample.
Finally, we can calculate the mass of sodium (Na) in the sample by multiplying the number of moles of Na by its atomic mass:
mass of Na = moles of Na × atomic mass of Na
mass of Na = 1.43 mol × 23.0 g/mol
mass of Na = 32.89 g
Therefore, there are approximately 32.89 grams of sodium in a 120-gram sample of NaHCO3.
Learn more about molar mass here : brainly.com/question/30459969
#SPJ1
A magnesium ion, Mg2+, hasA) 12 protons and 13 electrons. D) 24 protons and 22 electrons.B) 24 protons and 26 electrons. E) 12 protons and 14 electrons.C) 12 protons and 10 electrons.
Answers
A magnesium ion, Mg2+, has correct answer is option E) 12 protons and 14 electrons.
The correct answer is option E) 12 protons and 14 electrons. This is because the atomic number of magnesium, which is the number of protons in its nucleus, is 12. When it loses two electrons to become an ion, it still has 12 protons but now only 10 electrons. Therefore, the charge on the ion is 2+ (written as Mg2+). Options A, B, C, and D have incorrect numbers of protons and electrons for a magnesium ion.
Mg2+, an ion of magnesium, contains 12 protons and 14 electrons. This is so because magnesium has 12 protons, or its atomic number, in its nucleus. It still has 12 protons but only 10 electrons when it loses two electrons to become an ion. As a result, the ion has a 2+ charge, represented by the symbol Mg2+. For a magnesium ion, the protons and electrons in Options A, B, C, and D are in the wrong proportions.
To know more about magnesium ion click here:
https://brainly.com/question/1698012
#SPJ11
How much energy is required to raise the temperature of 3 kg of lead from 15°C to 20°C? Use the table below and this equation: Q = MCAT.
The question is written right above the table given.
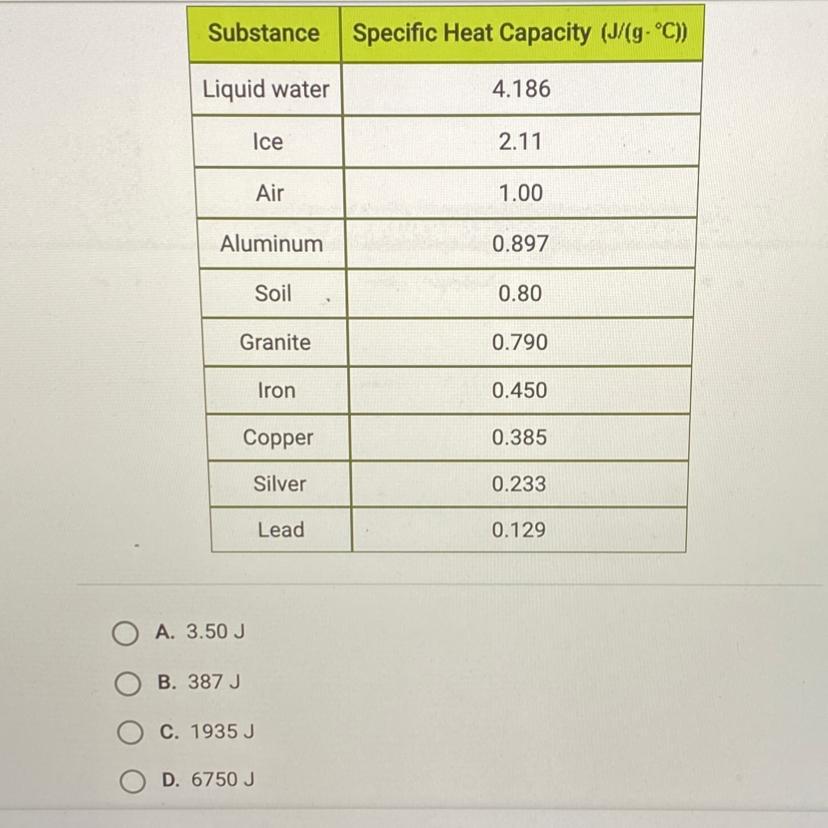
Answers
Answer:
1935J
Explanation:

Answer:
\(\boxed {\boxed {\sf C. \ 1935 \ J}}\)
Explanation:
The equation for this problem is:
\(q=mc\Delta T\)
where m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
The mass is 3 kilograms, but the specific heat capacity includes grams in the units. Convert kilograms to grams. There are 1000 grams in 1 kilogram.
\(\frac {1000 \ g}{1 \ kg}\)\(3 \ kg *\frac {1000 \ g}{1 \ kg}\)\(3 *1000 \ g = 3000 \ g\)The specific heat capacity for lead is found on the table. It is 0.129 J/g°C.
Let's find the change in temperature. It is raised from 15 °C to 20 °C.
\(\Delta T= final \ temperature - initial \ temperature \\\Delta T= 20 \textdegree C - 15 \textdegree C\\\Delta T= 5 \textdegree C\)Now we know every value.
m= 3000 g c= 0.129 J/g°CΔT= 5 °CSubstitute the values into the formula.
\(q= (3000 \ g)( 0.129 \ J/g \textdegree C)(5 \textdegree C)\)
Multiply the first 2 numbers together. The units of grams cancel.
\(q= (387 \ J/ \textdegree C )(5 \textdegree C)\)
Multiply again. This time the units of degrees Celsius cancel.
\(q= 1935 \ J\)
1935 Joules of energy are required and choice C is correct.
Which statement correctly compares the sun and planets? A. Planets have an atmosphere; the sun does not. B. Planets gives off energy; the sun does not. C. Planets orbit the sun; the sun is at the center of the solar system. D. Planets are all made of solid materials; the sun is made of gases.
Answers
Answer:
c
Explanation:
because the sun is at the center of the universe the planet orbit the sun its basically a simple answer so if you need more help im right here
The sun and the planets are part of the solar system. The sun is in the center of the system, and the planets revolve around it. Thus, option C is correct.
What is the solar system?A solar system is a part of the Milky Way galaxy that includes the planets, stars, sun, moon, asteroids, etc. The Sun is the star that is fixed at the center of the system.
The planets are not fixed and revolve in orbit around the sun and rotate on their axis. The sun and planets are made up of gases and have different atmospheres.
Therefore, option C. sun is at the center around which the planets revolve.
Learn more about the sun and planets here:
https://brainly.com/question/14029451
#SPJ2
a reaction mixture contains 0.108 atm of h2 , 0.049 atm of s2 , and 0.540 atm of h2s . determine how these conditions compare to equilibrium conditions. match the words in the left column to the appropriate blanks in the sentences on the right.
Answers
The appropriate blanks in the sentences on the right. Q> Kp. The system will respond by shifting to the left, or the side of the reactants.
What is meant by reactants?A reagent, also known as an analytical reagent, is a material or compound that is supplied to a system in order to trigger a chemical reaction or determine whether one has already occurred. Although the phrases "reagent" and "reactant" are frequently used interchangeably, the term "reactant" refers to the component that is consumed during a chemical reaction.Step 1: Information is provided
Kp = 2.4 * 10^-4
H2 has a partial pressure of 0.111 atm.
S2 is a partial pressure of 0.051 atm.
H2S partial pressure is equal to 0.566 atm.
Step 2: The equation that is balanced
2H2(g) + S2 = 2H2S(g) (g)
Step 3: Determine Q
Q is equal to (pS2 * (pH2) / (pH2S)2.
Q = (0.051 * 0.111²) / (0.566²)
Q = 0.00196 =1.96 *10^-3
Q> Kp
We have more products than reactants since Q>K. (pressure). The system will respond by shifting to the left, or the side of the reactants.
To learn more about reactants refer to:
https://brainly.com/question/26283409
#SPJ4
A substance with a pH of 11, how many more times more acidic than a substance with a pH of 1
Answers
A substance with a pH of 11 is 10 times more alkaline than a substance with a pH of 1.
What is pH and what is it for?The pH is a data that is used as a reference to classify chemical substances according to their alkalinity or acidity. The pH is measured on a scale from 0 to 14 that is divided as follows:
A pH value of 7 is neutral, which means that the substance or solution is neither acidic nor alkaline.A pH value of less than 7 means that it is more acidic.A pH value of more than 7 means that it is more alkaline.According to the above, it can be inferred that the substance that has a pH of 11 is not more acidic but alkaline. On the other hand, the substance with a pH of 1 is more acidic.
Learn more about pH in: https://brainly.com/question/15289741
#SPJ1
The balanced chemical equation for an acid-base reaction is
2HCI+ Ca(OH)2 +CaCl₂ + 2H₂O
For this reaction, how many water molecules form when x molecules of CaCl₂ form?
2
twice as many, 2x
half as many.
an equal number, x

Answers
The balanced chemical equation for the acid-base reaction is:
2HCl + Ca(OH)2 → CaCl2 + 2H2O
A balanced chemical equation is a representation of a chemical reaction that shows the relative number of reactant and product molecules involved. It follows the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. In a balanced equation, the number of atoms of each element on both sides of the equation is equal.
A balanced chemical equation includes chemical formulas of reactants on the left side of the arrow and the chemical formulas of products on the right side. Coefficients are used to balance the equation by adjusting the number of molecules or moles of each substance involved. These coefficients indicate the relative stoichiometric ratios between reactants and products.
According to the equation, for every 1 molecule of CaCl2 that forms, 2 water molecules are produced. Therefore, the correct answer is:
twice as many, 2x
For more details regarding balanced chemical equation, visit:
https://brainly.com/question/14072552
#SPJ1
Calculate the molar mass for oxygen gas
Answers
For example, the atomic mass of an oxygen atom is 16.00 amu; that means the molar mass of an oxygen atom is 16.00 g/mol. Further, if you have 16.00 grams of oxygen atoms, you know from the definition of a mole that your sample contains 6.022 x 10^23 oxygen atoms.
how many degrees of unsaturation are present in comppoiunds with a molecular formujla of c18h25f2in2o2
Answers
The compound with the molecular formula C18H25F2In2O2 has 4 degrees of unsaturation, which indicates the presence of multiple rings or pi bonds in the molecule.
To determine the degrees of unsaturation in a molecule, we need to calculate the molecule's hydrogen deficiency index (HDI) or index of hydrogen deficiency, which is the sum of the number of rings and pi bonds in the molecule.
The formula for HDI is:
HDI = (2n + 2 - x)/2
where n is the number of carbons and x is the sum of the hydrogens and halogens, plus any additional electrons from charges or unpaired electrons.
For C18H25F2In2O2, we have:
n = 18
x = 25 + 2 + 2 + 2 = 31
HDI = (2(18) + 2 - 31)/2 = 4
Therefore, there are 4 degrees of unsaturation in the compound with a molecular formula of C18H25F2In2O2. This suggests that the molecule may contain multiple rings or pi bonds, such as double or triple bonds, or a combination of both.
To learn more about degrees of unsaturation, refer:
https://brainly.com/question/30069703
#SPJ4
naoh is hygroscopic, meaning it absorbs water from the air. you can measure the amount of water it absorbs by taking its mass before and after removing the water in an oven (by the difference in mass before and after). would you use an analytical or top-loading balance for this?
Answers
It is better to use the analytical balance for smaller amounts of sodium hydroxide (up to a couple of grams).
The sensitivity of the analytical balance goes to a couple of tenths of a milligram, while the sensitivity of a top-loading balance goes to tens of milligrams at best. The amount of water absorbed by sodium hydroxide is significant, but not too great relative to the mass of the sample. With this in mind, it is far more recommendable to use the analytical balance over the top-loading balance in order to be able to register the change in mass at all.
You can learn more about sodium hydroxide here:
brainly.com/question/24010534
#SPJ4
I'll brainliest whoever has the best sentences in own words. I have my words!
explain how the amplitude, wavelength and frequency of sound waves are related to the qualities of sound that we can hear
Answers
Answer:
Waves have many properties, including frequency, wavelength, amplitude, timbre, and direction.A higher frequency sound is perceived as a higher note, because frequency is the same thing as pitch. The amplitude is how tall the wave is. A higher amplitude sound wave is a louder sound.
Explanation:
Explanation:
I'll brainliest whoever has the best sentences in own words. I have my words!
explain how the amplitude, wavelength and frequency of sound waves are related to the qualities of sound that we can hear
Stoichiometry Stumper
You are a forensic scientist. You are investigating a murder involving poison. The victim was poisoned with a compound called di-chloro benzene whose formula is C6H4Cl2. Autopsy results show that the victim’s body contained about 31 g of the poison, but the actual amount could have been slightly higher due to tissue absorption. The main suspect is his wife, Suzanne, who works as a chemistry professor at the local university. Records show that she purchased 15 g of benzene (C6H6) two days before the murder. Benzene is one of the compounds used to make the poison, but she claims she was using it to make ethyl benzene (C6H5CH3), an innocuous compound, for use in her lab. She shows you the bottle of ethyl benzene she claims to have made. It contains 25 grams of ethyl benzene.
Is she telling the truth or did she have more nefarious motives? If you can show that it is possible to produce 25 g of ethyl benzene from 15 grams of benzene, then she was telling the truth. Otherwise, you will have caught her in a lie, which makes it likely she killed her husband with the poison. After extensive research in the literature, you find the two reactions related to this case. Show all work.
Answers
From the calculation, Suzanne is lying and she is guilty of killing her husband.
What is stoichiometry?Stoichiometry provides a means of calculating the relationship between mass and mole or mole and volume in a reaction.
Now the preparation of ethylbenzene goes according to the reaction;
C6H6 + C2H6 ---->C6H5C2H5 + H2
Number of moles of benzene = 15 g/78 g/mol = 0.192 moles
Number of moles of ethyl benzene= 25 g/106 g/mol = 0.236 moles
Since the reaction is 1:1, 0.192 moles of ethyl benzene is produced.
Mass of 0.192 moles of ethylbenzene = 0.192 moles * 106 g/mol = 20.4 g
Suzanne is lying.
Learn more about stoichiometry: https://brainly.com/question/974398
How many mole are in 2.84*10^22 molecules of h2
Answers
Avogadro's Number and the Mole. The mole is represented by Avogadro's number, which is 6.022×1023 atoms or molecules per mol.
In the co-spotting portion of the thin-layer chromatography procedure, if your hypothesis is correct regarding the composition of your unknown dye solution, how many spots will you observe in the middle lane after elution?
Answers
In the co-spotting portion of thin-layer chromatography (TLC), if your hypothesis is correct regarding the composition of your unknown dye solution, you would expect to observe a single spot in the middle lane after elution.
During TLC, the sample mixture is applied as spots on the TLC plate, and as the solvent moves up the plate through capillary action, the individual components of the mixture separate based on their affinity for the stationary phase (adsorbent) and the mobile phase (solvent).
If your hypothesis is correct and the unknown dye solution consists of a single compound or a mixture of compounds that have similar affinities for the adsorbent and solvent system used in TLC, you would expect to see only one spot at the end of the elution process in the middle lane.
However, it's important to note that if your hypothesis is incorrect and the unknown dye solution is a mixture of compounds with different affinities, you may observe multiple spots in the middle lane corresponding to the individual components present in the mixture. TLC can help in identifying different components and their relative proportions in a mixture based on the number and position of the spots obtained after elution.
Learn more about Thin Layer chromatography here: https://brainly.com/question/14219599
#SPJ11
Which phrase describes a metamorphic rock?
formed from a volcano
was once buried underground
formed from evaporating water
contains minerals that have not changed
Answers
Answer:
C: was once buried underground
Explanation:
Hope this helps!
Answer:
The correct answer you are looking for is B i have got 100% on this test.
Explanation:
hop this helps! └(^o^)┘ ↖(^ω^)↗
why is citric acid added to food?to add colorto add tartnessto add bitternessto add sweetness
Answers
Citric acid is added to food to add tartness and enhance the flavor. The correct option is b.
Citric acid, a natural compound found in citrus fruits, is commonly added to food for its tart flavor and ability to enhance taste. Here's a step-by-step explanation:
1. Tartness: Citric acid is highly acidic and has a sour taste. When added to food, it provides a sharp, tangy flavor that adds tartness. This tartness can help balance the overall taste profile of a dish, especially in sweet or savory recipes.
2. Flavor enhancement: Citric acid acts as a flavor enhancer, intensifying the existing flavors in food. It has the ability to enhance the perception of other taste sensations, such as sweetness and saltiness, making food taste more vibrant and flavorful.
3. Preservation: Citric acid also acts as a natural preservative in some food products. It has antimicrobial properties that inhibit the growth of certain bacteria and fungi, helping to extend the shelf life of foods and prevent spoilage.
4. pH adjustment: Citric acid can be used to adjust the pH level of certain food products. It is commonly used in canning and preserving processes to create an acidic environment that inhibits bacterial growth and helps maintain product quality and safety.
Overall, the addition of citric acid to food primarily serves to enhance flavor, provide tartness, and potentially contribute to preservation. Option b is the correct one.
To know more about Citric acid refer here:
https://brainly.com/question/28266073#
#SPJ11