Given the following equation:
2H2O --> 2H2 +O2
What mass of oxygen would form from 5 moles of water?
Question 6 options:
.078
320
.3125
80
Answers
Related Questions
PLEASE HELP!!
How many moles are present in 431.56 grams of sodium bicarbonate ( NaHCO3)?
Molar mass of sodium bicarbonate (NaHCO3) = 84.01 g/mol
Answers
Answer:
5.14mole
Explanation:
Given parameters:
Mass of sodium bicarbonate = 431.56g
Unknown:
Number of moles = ?
Solution:
To find the number of moles, we use the expression below:
Number of moles = \(\frac{mass}{molar mass}\)
Molar mass = 84.01g/mol
Number of moles = \(\frac{431.56}{84.01}\) = 5.14mole
Help please help me!!!!!
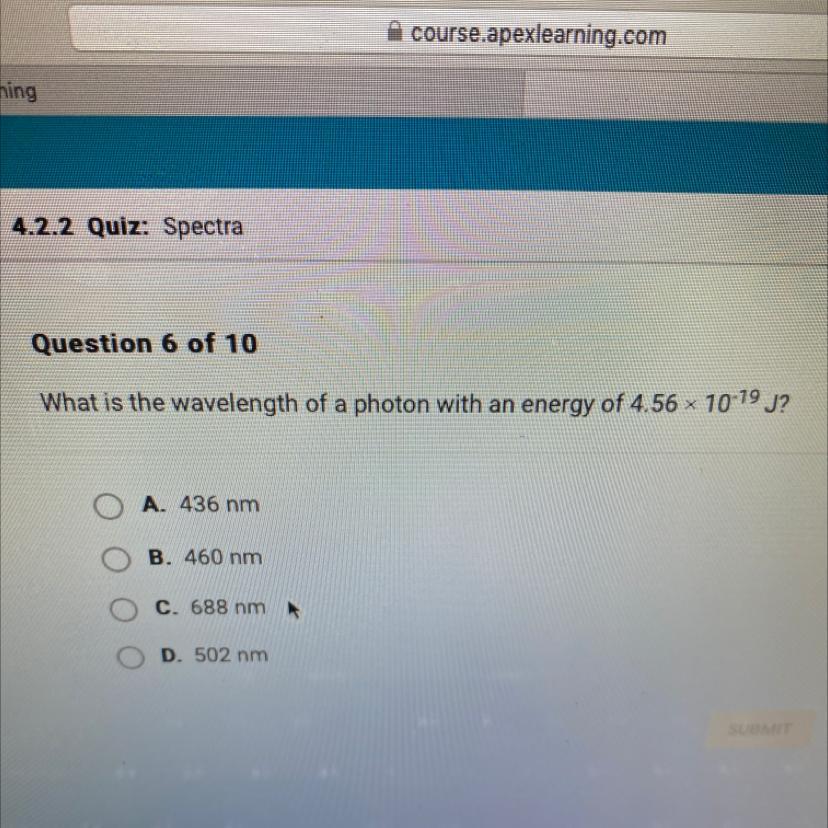
Answers
Answer:
B. 460 NM
Explanation:
THAT THE ANSWER
why is it important to keep the cuvettes at a consistent distance from the lamp as you perform this activity?
Answers
By keeping the cuvettes at a consistent distance from the lamp, both photosynthesis and cellular respiration have had an equal chance to thrive.
Why is it necessary that the temperature of the pondweed remain constant?Describe the significance of maintaining a steady temperature for the pondweed.Since enzymes are responsible for regulating photosynthesis, temperature has an impact on how quickly it occurs. High temperatures can cause enzymes to become inactive.
When grown in light, what process does the algae carry out?In the absence of sunshine, some cells use a sequence of chemical events called photosynthesis to convert water and carbon dioxide into sugars.As a byproduct, oxygen gas is created.In eukaryotic cells, which include plants and many parasitoid wasps like green algae, photosynthesis takes place in chloroplasts.
To know more about photosynthesis visit:
https://brainly.com/question/1388366
#SPJ1
A particular saturated solution of Ca3(PO4)2 has [Ca2+]=[PO3?4]=2.9×10?7M. A) What is the value of Ksp for Ca3(PO4)2?
Answers
The Ksp value of \(Ca_3(PO_4)_2\) is \(2.9 * 10^{-14 }M^2.\)
Ksp stands for "equilibrium constant for a speciation reaction." It is a measure of the stability of a solution with respect to the formation of a particular ionic species. Specifically, it is the ratio of the concentrations of the ions that are consumed (or "used up") in forming the ionic species to the concentration of the ionic species itself.
Ksp values are used to predict the relative stability of different ionic species in a solution, and they can be used to predict the concentrations of the ions in a solution as a function of time, given certain conditions. Ksp values can also be used to predict the conditions under which a particular ionic species will be formed or consumed in a reaction
The Ksp value of \(Ca_3(PO_4)_2\) can be calculated using the following equation:
\([Ca_2+][PO_3^4] = Ksp\)
From the given information, we have\([Ca_2+] = 2.9 * 10^{-7 }M, [PO_3^4] = 2.9 * 10^{-7 }M.\)
Therefore, we can substitute these values into the equation and solve for Ksp:
\([Ca__2+][PO3^4] = {(Ksp)} \\\\\\2.9 * 10^{-7} M[Ca_2+] * 2.9 * 10^{-7} M[PO_3^4] = Ksp\\\)
Ksp = \(2.9 * 10^{-14 }M^2.\)
Therefore, the Ksp value of \(Ca_3(PO_4)_2\) is \(2.9 * 10^{-14 }M^2.\)
Learn more about Ksp value visit: brainly.com/question/29610286
#SPJ4
Which would aid a student in preparedness for a lab?
O using a fire extinguisher if there is a fire
O reading the lab manual before class
O telling the teacher about a chemical spill
O cleaning up after the lab
Answers
Answer: cleaning up after the lab, D.
Explanation:
4. When the mole fraction of solute is 1, there is
(a) a 1:1 ratio of solute to solvent.
(b) no solute present.
(c) only solute present.
(d) only solvent present.
(e) 1 mole of solute and 99 moles of solvent.
Answers
Answer:
(c) only solute present
Explanation:
In chemistry, the mole fraction, denoted by X, refers to the number of moles of a substance in a compound/mixture divided by the total number of substances in the same compound or mixture.
In this case, we can say that mole fraction represents the number of solutes to the number of solutes and solvent in the solution i.e. X = nA/nA + nB
Where; nA = number of solutes
nB = number of solvent
X = mole fraction.
Based on this analogy, When the mole fraction of solute is 1, there is only solute present. That is; X = 1 / 1 + 0
X = 1/1 = 1.
i am pouring concentrated sulfuric acid from a 1-gallon container. i need to use the following ppe for protection against potential splash of a corrosive liquid.
Answers
Personal protective equipments to be worn while handling corrosive liquids are safety goggles, hand gloves and closed toed shoes.
What are personal protective equipments ?Personal protective equipment is a protective clothing which is worn to protect the wearer's body from hazard or injury.The hazards which can be addressed by the use of personal protective equipment are physical,chemical and bio hazards.
It imposes a barrier between the user and the working environment.The main purpose of personal protective equipment is to reduce exposure of employees to the hazards.
It has a limitation that it does not eliminate the hazard and may lead to harm to the employee if the equipment is damaged.
Learn more about personal protective equipment,here:
https://brainly.com/question/28900790
#SPJ1
Why does a food web have more biodiversity?
Answers
Answer:
Biodiversity is important to the stability of food webs because it increases the complexity of interactions between organisms and makes them better :')
What is the volume of a canister filled with gas if its pressure is changed to 9.8 atm from 2.5 atm at a volume of 9.1 L?
Answers
Answer:
2.3 L
Explanation:
A change in pressure-volume at constant temperature is described by the Boyle's law. The mathematical relationship between initial pressure and volume (P₁ and V₁) and final pressure and volume (P₂ and V₂) is given by:
P₁V₁ = P₂V₂
We have the following data:
initial pressure: P₁= 2.5 atm
initial volume: V₁ = 9.1 L
final pressure: P₂= 9.8 atm
Thus, we introduce the data in the mathematical expression and calculate the final volume V₂, as follows:
V₂ = P₁V₁/P₂ = (2.5 atm x 9.1 L)/9.8 atm = 2.3 L
Therefore, the volume of the canister is 2.3 L.
A story that explains something in nature is called a_______
Answers
Answer: myth .
Explanation: traditional story, especially one concerning the early history of a people or explaining a natural or social phenomenon, and typically involving supernatural beings or events.
"ancient Celtic myths"
usually traditional story of ostensibly historical events that serves to unfold part of the world view of a people or explain a practice, belief, or natural phenomenon creation myths. b : parable, allegory Moral responsibility is the motif of Plato's myths.
Question 1 Ethylbenzene and toluene can be separated from each other by flash distillation. Their vapor pressures can be described using the following Antoine-equation: 10 log P(bar) = A-TUK)+C with for toluene
a) A boiling liquid feed (T= 65 °C), which contains 55 mol% ethylbenzene. is flash distilled, whereby 40% of the feed is evaporated. What are the compositions of the streams leaving the flash-drum?
b) A boiling liquid feed (p 0.20 bar), which contains 55 mol% ethylbenzene, is flash distilled, whereby the liquid stream leaving the flash vessel is enriched to 62 mol ethylbenzene. What is the ratio of the streams leaving the flash-drum VIL)?
Answers
In both cases, the Antoine equation constants (A, B, C) for ethylbenzene and toluene should be provided for accurate calculations.
(a) Solve for the mole fractions of each component in the liquid phase:
x(EB)_liquid = x(EB) / (1 - α + α * (P(EB)_vapor / P(EB)_liquid))
x(TOL)_liquid = x(TOL) / (1 - α + α * (P(TOL)_vapor / P(TOL)_liquid))
(b) Solve for the mole fractions of each component in the liquid phase:
x(EB)_liquid = x(EB) / (1 + ((P(EB)_vapor - P(EB)_liquid) / P(total)))
x(TOL)_liquid = x(TOL) / (1 + ((P(TOL)_vapor - P(TOL)_liquid) / P(total)))
To solve these flash distillation problems, we need to use the vapor-liquid equilibrium (VLE) data provided by the Antoine equation. Let's solve each part of the question separately:
a) Flash distillation with 40% evaporation:
Given:
Boiling liquid feed temperature (T) = 65 °C
Feed composition: 55 mol% ethylbenzene (EB) and 45 mol% toluene (TOL)
Evaporation fraction (α) = 40% = 0.4
We'll assume the total pressure remains constant at the boiling point temperature.
Calculate the vapor pressure of each component at the given temperature using the Antoine equation:
For ethylbenzene:
10 log P(EB) = A - B / (T + C)
10 log P(EB) = A - (B / (T + C))
For toluene:
10 log P(TOL) = A - (B / (T + C))
Calculate the partial pressure of each component in the liquid feed:
P(EB)_liquid = P(total) * x(EB)
P(TOL)_liquid = P(total) * x(TOL)
Calculate the partial pressure of each component in the vapor phase:
P(EB)_vapor = α * P(EB)_liquid
P(TOL)_vapor = α * P(TOL)_liquid
Use the Antoine equation to solve for the mole fractions of each component in the vapor phase:
P(EB)_vapor = P(total) * y(EB)
P(TOL)_vapor = P(total) * y(TOL)
Solve for the mole fractions of each component in the liquid phase:
x(EB)_liquid = x(EB) / (1 - α + α * (P(EB)_vapor / P(EB)_liquid))
x(TOL)_liquid = x(TOL) / (1 - α + α * (P(TOL)_vapor / P(TOL)_liquid))
b) Flash distillation with enriched liquid stream:
Given:
Boiling liquid feed pressure (P) = 0.20 bar
Feed composition: 55 mol% ethylbenzene (EB) and 45 mol% toluene (TOL)
Liquid stream leaving the flash vessel composition: 62 mol% ethylbenzene (EB) and 38 mol% toluene (TOL)
Calculate the vapor pressure of each component at the boiling point temperature using the Antoine equation.
Calculate the total pressure in the flash drum using Dalton's law:
P(total) = P(EB)_vapor + P(TOL)_vapor
Calculate the partial pressure of each component in the liquid feed:
P(EB)_liquid = P(total) * x(EB)
P(TOL)_liquid = P(total) * x(TOL)
Use the Antoine equation to solve for the mole fractions of each component in the vapor phase:
P(EB)_vapor = P(total) * y(EB)
P(TOL)_vapor = P(total) * y(TOL)
Solve for the mole fractions of each component in the liquid phase:
x(EB)_liquid = x(EB) / (1 + ((P(EB)_vapor - P(EB)_liquid) / P(total)))
x(TOL)_liquid = x(TOL) / (1 + ((P(TOL)_vapor - P(TOL)_liquid) / P(total)))
Note: In both cases, the Antoine equation constants (A, B, C) for ethylbenzene and toluene should be provided for accurate calculations.
To know more about the word distillation, visit:
https://brainly.com/question/31829945
#SPJ11
a. What is the electron-domain (charge-cloud) geometry of BrF5? Enter the electron-domain geometry of the molecule.
b. What is the molecular geometry of BrF5?
c. Ignoring lone-pair effects, what is the smallest bond angle in BrF5?
d. Which choice best describes the polarity of BrF5?
The molecule is polar and has polar bonds.
The molecule is nonpolar and has polar bonds.
The molecule is polar and has nonpolar bonds.
The molecule is nonpolar and has nonpolar bonds.
e. Of the molecules below, only ________ is polar.
H2
SiS2
CH4
AsH3
PF5
Answers
(a) Electron domain geometry - Octahedral.
(b) Molecular geometry - Square pyramidal.
(c) Smallest bond angle - 90°
(d) The polarity of BrF₅, best choice is: The molecule is polar and has polar bond.
(e) AsH₃ is polar molecule.
What is polar and non-polar molecule?A molecule's valence shell electrons are shown in a very simple manner via a Lewis structure. It serves as a visual representation of how the electrons surrounding certain molecules' atoms are positioned.
It demonstrates the bonds that exist between a molecule's atoms and its lone pairs of electrons. When used in conjunction with hybrid orbitals, Lewis structures can also be helpful in predicting molecular geometry.
(a) Electron domain geometry - Octahedral.
(b) Molecular geometry - Square pyramidal.
(c) Smallest bond angle - 90°
(d) The polarity of BrF₅, best choice is: The molecule is polar and has polar bond.
(e) AsH₃ is polar molecule.
To know more about geometry refer to:
https://brainly.com/question/20274710
#SPJ1
a galvanic cell zn | zn²⁺ || ni²⁺ | ni runs spontaneously. if a current is imposed to turn this into an electrolytic cell, which of the following will occur?1 point
Answers
Answer:
Zn²⁺ gets reduced
Explanation:
An electrolytic cell runs in the opposite direction of a galvanic cell. Applying a current to the galvanic cell Zn | Zn²⁺ || Ni²⁺ | Ni would convert the Zn anode to a cathode and cause Zn²⁺ to be reduced.
6. An iron nail is kept in each of the following liquids. In which case does it lose shine and appear dull a. mustard oil b.kerosen c. soft drink
Answers
Answer:
Answer is 'b' Soft drink
Explanation:
An iron nail kept in a soft drink will lose its shine and appear dull. Because mustard oil, coconut oil, and kerosene have no reaction with iron nails, they do not lose their shine when maintained in these solutions.
if ocean water tempratures cooled to 20 degree c what impact would this have on the weather system formation?
PLSSS I NEED IT FAST
Answers
Given the standard enthalpy changes for the following two reactions
Given the standard enthalpy changes for the following two reactions:
(1) 2C(s) + 2H2(g)C2H4(g)...... ΔH° = 52.3 kJ
(2) 2C(s) + 3H2(g)C2H6(g)......ΔH° = -84.7 kJ
what is the standard enthalpy change for the reaction:
(3) C2H4(g) + H2(g)C2H6(g)......ΔH° = ?
Answers
The standard enthalpy change for reaction (3) is 117.1 kJ.
The standard enthalpy change for reaction (3) can be calculated by using the enthalpy changes of reactions (1) and (2) and applying Hess's Law.
To do this, we need to manipulate the given equations so that the desired reaction (3) can be obtained.
First, we reverse reaction (1) to get the formation of C2H4(g) from C2H6(g):
C2H4(g)C2H6(g) ΔH° = -52.3 kJ
Next, we multiply reaction (2) by 2 and reverse it to obtain 2 moles of C2H6(g) reacting to form 3 moles of H2(g):
2C2H6(g)2C(s) + 3H2(g) ΔH° = 169.4 kJ
Now, we add the two modified equations together:
C2H4(g)C2H6(g) ΔH° = -52.3 kJ
2C2H6(g)2C(s) + 3H2(g) ΔH° = 169.4 kJ
When adding these equations, the C2H6(g) on the left side cancels out with the C2H6(g) on the right side, leaving us with the desired reaction (3):
C2H4(g) + H2(g)C2H6(g) ΔH° = -52.3 kJ + 169.4 kJ = 117.1 kJ
Learn more about standard enthalpy here :-
https://brainly.com/question/28303513
#SPJ11
English chemistry problem (easy) I will select the best one, Please quickly do it

Answers
Answer:
An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.”
Explanation:
this should help
Find the volume in milliliters (ml) of 25gof benzene. The density of benzene is 0.8765 g/mL
Answers
Answer:
28.52 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \\\)
From the question we have
\(volume = \frac{25}{0.8765} \\ = 28.52253...\)
We have the final answer as
28.52 mLHope this helps you
Which is an example of a polymer?
A. diamond
B. carbon monoxide
C. sodium chloride
D. cellulose
Answers
Answer:
\(d)cellulose \\ monomer \: is \: glucose \\ thank \: you\)
Answer:
cellulose
Explanation:
Explain why for static electricity to occur between two surfaces that are rubbed against each other, one of the materials has to be an insulator
Answers
When two surface come in contact and rub each other a frictional force causing them s deformation and this leads to a voltage giving rise to a static charge.
What is static charge?Static charge is developed within a body temporarily with an induced polarization. These charges are not moving and set into a pole in the material.
When two materials rub against each other in which one is a conductor and other to be an insulator, they will attract by the induced polarization. Rubbing causes the free electrons in the condutor to be transferred towards the insulator.
This will cause a deformation that, the random charges in the material get polarized where the electrons in the insulator will repel to the opposite pol and positive charges will align in a pole close to the condutor.
This charge separation causes the positive pole of the insulator gets attracted into the conductor surface. Hence, there forms a static electricity by the passage of electron.
To find more on static electricity, refer here:
https://brainly.com/question/12791045
#SPJ5
2. I give energy to living things. Who am I? Ans:
Answers
sunlight gives energy to living things
Explanation:
sun is the main energy for the earth
Explain why is ortho nitrophenol more acidic than ortho methoxyphenol
Answers
Answer:
Ortho nitrophenol is more acidic than ortho-methoxyphenol.
Reason:
This is because the nitro-group is an electron-withdrawing group. In the case of ortho methoxyphenol; methoxy group is an electron-releasing group. Thus, it increases the electron density in the O−H bond and hence, the proton cannot be given out easily.
Hope this helps you. Do mark me as brainliest.
carbon + oxygen -->
carbon dioxide
balancing equation
Answers
Carbon = C
Oxygen = O
CO2 = Carbondioxide
Balanced equation :
C + O2 = CO2
Answer:
co2
Explanation:
c+o2-------------> co2
Predict the products for the single replacement reactions given. check to see that the equations are balanced. ca mgcl2 → ? cacl2 mg ca cl2 mg cl2 cacl mg
Answers
When a less active ingredient in a molecule can be changed with a more reactive element, we forecast that just one reaction will take place. Ca + MgCl₂ → CaCl₂ + Mg will be the chemical equation for a fair system.
What are equations in science?An illustration of a chemical that is often expressed as a linear array with an arrow or a group of arrows separating the symbols and amounts of the reactants and those of the products.
The given equation is Ca + MgCl₂ →
It is well known that when calcium (Ca) reacts with magnesium chloride (MgCl₂), calcium chloride (CaCl₂) and magnesium are produced.
Ca + MgCl₂ → CaCl₂ + Mg.
Calcium only has atom on the photocatalyst surface and one on the product side, as can be seen. Chlorine, from the other hand, has one atom on both the reactant and product sides.
As a result, the response will be balanced.
To know more about Equations visit:
https://brainly.com/question/28774454
#SPJ4
The correct chemical formula for the compound tin (II) carbonate is _____. Select one: a. Sn2CO3 b. Sn2(CO3)2 c. Tn2CO3 d. SnCO3
Answers
Answer:
the answer would be D, snC03
How large a net force is required to accelerate a 2000-kg suv from rest with an acceleration of 0. 14 m/s2?.
Answers
A large net force of 280N is required to accelerate a 2000 kg suv from rest with an acceleration of 0.14m/s2 .
According to Newton’s 2nd law of motion, force is equal to the change in momentum per change in time.Acceleration of an object depends on the mass of the object and the amount of force applied.
Force=mass* acceleration
Here, mass is equal to 2000 kg and acceleration is equals to 0.14m/s/s.putting these values,
F= ma
F= 2000kg * 0.14 m/s2
= 280 kg m/s2
=280N
So, 280N of force is required.
To learn more about newton's 2nd law please visit:
https://brainly.com/question/25545050
#SPJ4
What mass of K2SO4 must be added to 1.20 liters of water to produce a 1.50 M solution?
Answers
Answer:
313.2 g of \({K_{2} }S O_{4}\) must be added to 1.20 liters of water to produce a 1.50 molar solution.
Explanation:
What is molarity?
Molarity is a unit of concentration of a solution. It is defined by the number of moles of the solute that is present in one liter (1L) of the solution. It is denoted by M. Thus, molarity = \(\frac{Number of moles of the solute (n) }{Volume of the solution (V) (in L)}\)∴ The number of moles of solute = molarity x volume of the solution.According to the given question,
Molarity of the solution = 1.50 MThe volume of the solution = 1.20 LUnknown = Mass of \({K_{2} }S O_{4}\) required.Solution :
∴ Number of moles of solute, here, \({K_{2} }S O_{4}\)
= molarity x volume of the solution
= 1.20 x 1.50 = 1.8
∴ Mass of 1.8 moles of \({K_{2} }S O_{4}\) = 1.8 x molar mass of \({K_{2} }S O_{4}\)
Now the molar mass of \(K_{2} SO_{4}\)
= (Gram atomic mass of K x 2) + (Gram atomic mass of S) + (Gram atomic mass of O x 4)
= (39x2) + 32 + (16 x 4) g
= 174 g.
∴ Mass of 1.5 moles of \({K_{2} }S O_{4}\)
= 1.8 x molar mass of \({K_{2} }S O_{4}\)
= 1.8 x 174 g
= 313.2 g.
Thus, 313.2 g of \({K_{2} }S O_{4}\) must be added to 1.20 liters of water to produce a 1.50 M solution.
To know more about molarity, visit :
https://brainly.com/question/15406534
1. How many sublevels are in the following principal energy levels?
a. n=1
b. n=2
c. n=3
d. n=4
e, n=5
f. n=6
Answers
Explanation:
a 1 ( only "s"
b 2 ( "s" ,"p" )
c 3 ( "s" , "p" , "d")
d 4 ( "s", "p" , "d", "f")
e 5 ( "s", "p", "d", "f", "g")
f 6 ( "s", "p", "d", "f", "g", "h" )
The number of atoms of any element in the given chemical formula is the number that is written on the food of the symbol of that element. Therefore, sublevels are present in the principal energy levels.
What is atom?Atom is the smallest particle of any matter. Atom combines to form element and element combine to form molecule or compound.
Atom consists of electron, proton and neutron. The total mass of atom is inside the nucleus. Inside the nucleus proton and neutron is there. So calculate mass of an atom, total mass of all protons is added to the total mass of neutron. Electrons revolve around the nucleus.
principal energy levels sublevels
1 s
2 s,p
3 s,p,d
4 s,p,d,f
5 s,p,d,f,g
6 s,p,d,f,g,h
Therefore, sublevels are present in the principal energy levels.
Learn more about atoms, here:
https://brainly.com/question/13518322
#SPJ2
Someone plz help I Don’t know I would do something like this and I really need to get it done
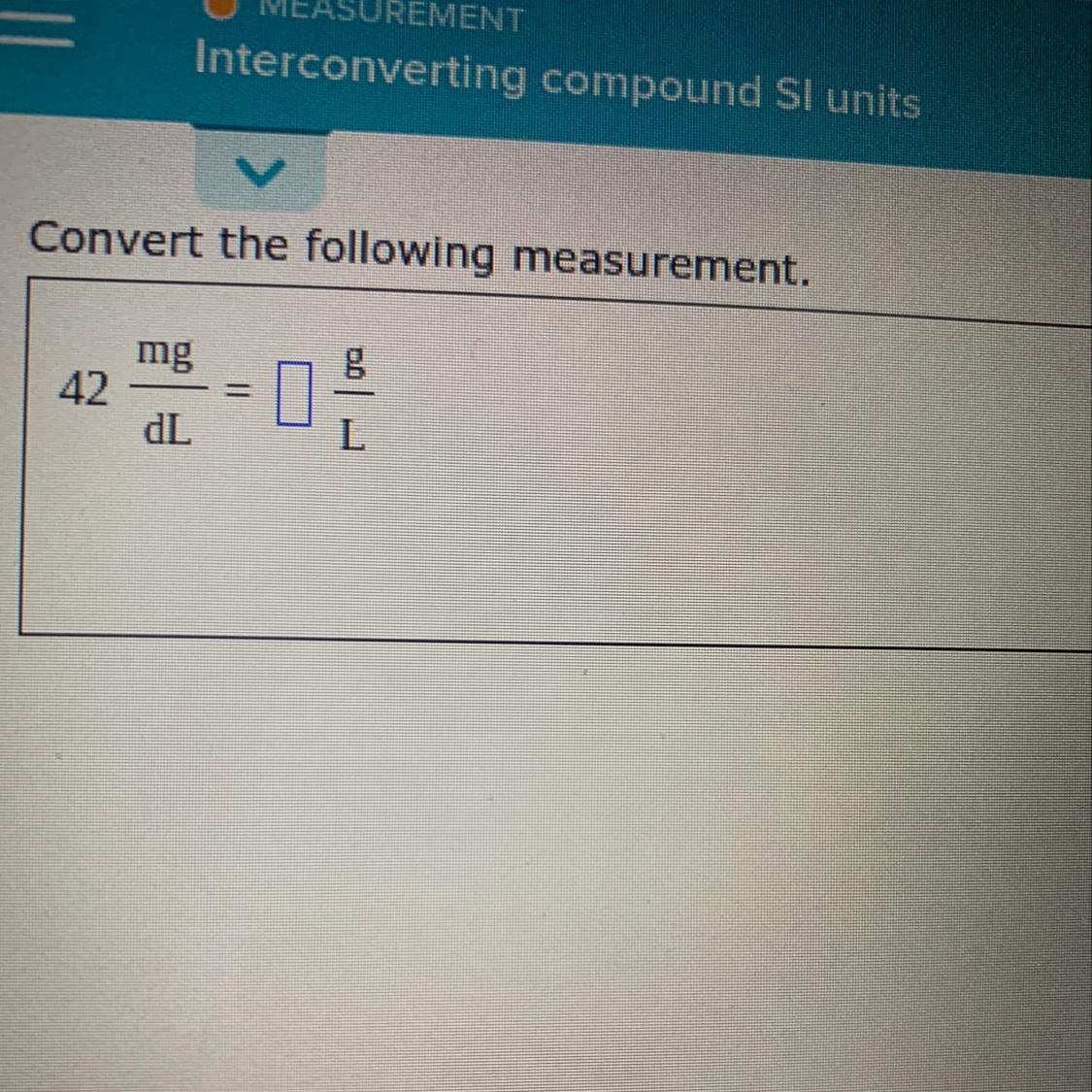
Answers
1dL =.1L
0.001/.1 *42=0.42
PLEASE HELP ME ASAP
