Formic acid is as a preservative and antibacterial agent in livestock feed. the pka of formic acid is 3.75. what is the reaction of formic acid (ch2o2) with water?
Answers
The reaction of formic acid (CH2O2) with water involves the dissociation of formic acid into its ions, hydrogen ions (H+) and formate ions (HCOO-). This process is known as ionization.
Formic acid is a weak acid, meaning that it does not fully dissociate in water. Instead, only a small fraction of formic acid molecules ionize. The degree of ionization is determined by the acid's pKa value. The pKa of formic acid is 3.75, which indicates that it is a moderately weak acid.
In the presence of water, formic acid reacts as follows:
CH2O2 + H2O ⇌ H+ + HCOO-
When formic acid dissolves in water, some molecules break apart, releasing hydrogen ions (H+) and formate ions (HCOO-) into the solution. The hydrogen ions give the solution its acidic properties.
The equilibrium arrow (⇌) in the reaction equation indicates that the reaction is reversible. This means that some formic acid molecules can also combine with hydrogen ions and formate ions to reform the original formic acid molecules.
It's important to note that the extent of ionization depends on the concentration of formic acid and the pH of the solution. At low pH values, where the concentration of hydrogen ions is high, the ionization of formic acid increases. At higher pH values, the ionization decreases.
Understanding the reaction of formic acid with water is crucial in evaluating its preservative and antibacterial properties in livestock feed. The acidic nature of formic acid helps inhibit the growth of bacteria and fungi, thereby preserving the feed and preventing spoilage.
Overall, the reaction of formic acid with water involves the partial dissociation of formic acid into hydrogen ions and formate ions, contributing to its role as a preservative and antibacterial agent in livestock feed.
Learn more about ionization here:-
https://brainly.com/question/1602374
#SPJ11
Related Questions
At which location are metamorphic rocks most likely to
form?
ОА
ов
с C
OD
the answer is B
Answers
Answer:
Yup B
Explanation:
6. When cooking an egg and waiting for coagulation, what are two things to look for? (1 point)
Answers
When cooking an egg and waiting for coagulation, two things to look for are the firmness of the egg white and the doneness of the yolk.
The egg white should become opaque and set, indicating that it has coagulated properly. The yolk can be cooked to different degrees of doneness, depending on personal preference. For a runny yolk, it should still be soft and slightly jiggly in the center. For a firmer yolk, it should be more set and less jiggly. By observing these two aspects, you can determine the coagulation stage of the egg and achieve the desired texture for your egg dish.
To know more about coagulation, visit:
https://brainly.com/question/31116523
#SPJ11
A sample of NH3, gas occupies 75.0 liters at STP. How many molecules is this?
Answers
Answer:
2.016E24 same as 2.016 × 10^24
Explanation:
we have to use 2 conversion facts
22.4 and 6.02E23
1. Convert to moles of NH3 from liters of NH3
75.0 ÷ 22.4 = 3.348 moles of NH3
2. Convert to molecules of NH3 from moles of NH3
3.348 × 6.02E23 = 2.016E24 molecules of NH3
please let me know if i need to further eloborate :)
Use the tabulated half-cell potentials to calculate ΔG° for the following balanced redox reaction. Pb2+(aq) + Cu(s) → Pb(s) + Cu2+(aq)
Answers
Answer:
90.71 KJ
Explanation:
From the reaction equation, lead is the cathode while copper was the anode.
Hence;
E°anode = +0.34V
E°cathode = -0.13 V
E°cell =E°cathode - E°anode
E°cell = -0.13 V - 0.34V
E°cell = -0.47 V
But;
ΔG° = -nFE°cell
n= number of electrons transferred = 2
F = Faraday's constant = 96500 C
E°cell = -0.47 V
ΔG° = -(2 * 96500 * (-0.47))
ΔG° = 90,710 J or 90.71 KJ
The standard Gibbs free energy for the reaction
Pb²⁺(aq) + Cu(s) → Pb(s) + Cu²⁺(aq) is 89.3 kJ/mol.
Let's consider the following redox reaction.
Pb²⁺(aq) + Cu(s) → Pb(s) + Cu²⁺(aq)
We can identify both half-reactions.
Reduction (Cathode): Pb²⁺(aq) + 2 e⁻ → Pb(s) E°red = -0.126 V
Oxidation (Anode): Cu(s) → Cu²⁺(aq) + 2 e⁻ E°red = +0.337 V
We can calculate the standard potential of the cell (E°cell) using the following expression.
E°cell = E°red,cathode - E°red,anode = -0.126 V - 0.337 V = -0.463 V
The standard Gibbs free energy (ΔG°) is a way to measure the spontaneity of a reaction. We can calculate it using the following expression.
ΔG° = − n × F × E°cell =
ΔG° = − 2 mol × (96,485 J/V.mol) × (-0.463 V) × (1 kJ/1000 J) = 89.3 kJ/mol
where,
n are the moles of electrons involvedF is Faraday's constantSince ΔG° > 0, the reaction is not spontaneous.
The standard Gibbs free energy for the reaction
Pb²⁺(aq) + Cu(s) → Pb(s) + Cu²⁺(aq) is 89.3 kJ/mol.
Learn more about Gibbs free energy: https://brainly.com/question/9552459
How does Lori make the solution?

Answers
Answer:
I would say the top one but im not confident in the answer
someone help me please....
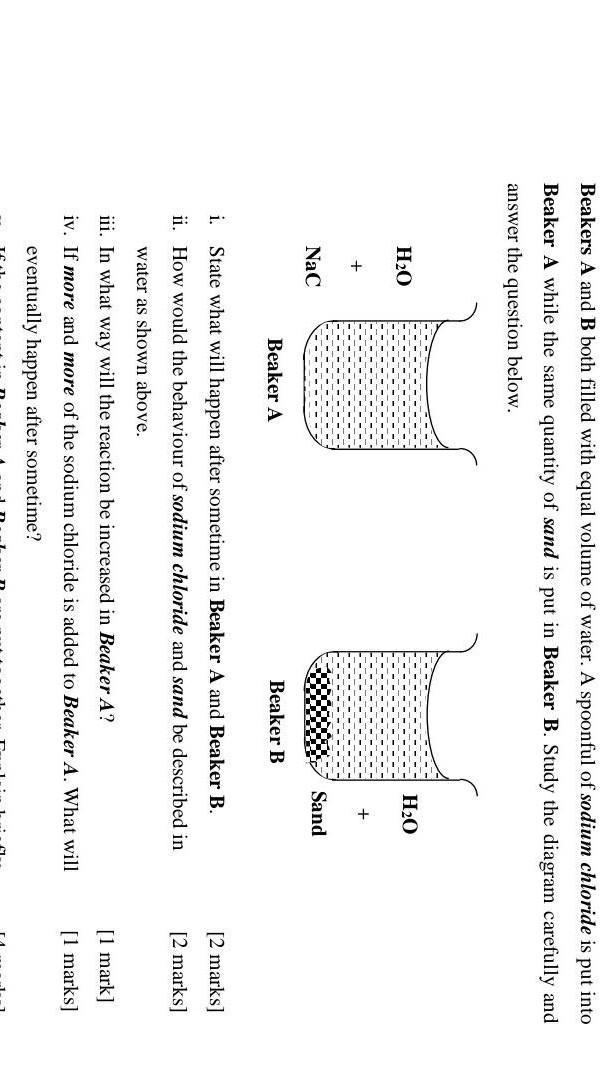
Answers
Answer:
i- In beaker A, sodium chloride will dissolve with water.
and in beaker B, there will be no reaction.
ii- sodium chloride is soluble in water, while sand is insoluble.
iii- The reaction can be increased by adding more sodium chloride to the beaker.
iv- The sodium chloride will no longer dissolve in the water.
Sodium-24 is a radioactive isotope with a half life of 14.8 days. Radioactive decay is a first order process. How many half-lives are required for a sample of Na-24 to decay toth of its initial 1/256th concentration.
(A) 5
(B) 6
(C) 7
(D) 8
(E) 4
Answers
(B) 6.A sample of Na-24 needs 6 half-lives to degrade to 1/256th of its starting concentration.
The formula for radioactive decay is as follows:
\(N(t) = N_{0}e^{(-\lambda t)}\)
Where N(t) is the amount of radioactive material remaining at time t, N₀ is the initial amount of radioactive material, and λ is the decay constant.
The half-life of a radioactive material is defined as the time period after which half of the initial amount of radioactive material has decayed. This is given by the equation:
\(T_\frac{1}{2}= ln\frac{2}{\lambda}\)
For Sodium-24, the half life is 14.8 days, so the decay constant is given by:
\(\lambda = ln\frac{2}{T_\frac{1}{2} }= ln\frac{2}{14.8} = 0.04793\)
To find the number of half-lives required for a sample of Na-24 to decay to 1/256th of its initial concentration, we can rearrange the equation for radioactive decay and solve for t:
\(t = ln(\frac{\frac{N_0}{N(t)}}{\lambda})\)
Since we know that \(N(t) = \frac{N_0}{256}\), we can substitute N(t) into the equation:
\(t = ln(\frac{\frac{N_0}{(\frac{N_0}{256})}}{\lambda})\)
\(t = ln\frac{(256)}{\lambda }\)
\(t = ln\frac{(256)}{0.04793}\)
t = 5.86
The right response is 6 half-lives , as the number of half-lives must be an integer.
learn more about radioactive decay refer:brainly.com/question/1770619
#SPJ1
among the trace minerals, which mineral is found in highest concentrations in the body? among the trace minerals, which mineral is found in highest concentrations in the body? zinc copper fluoride iron
Answers
The correct answer is option D.
Among the trace minerals, iron is the mineral that is found in highest concentrations in the body.
In human body, there are 21 trace minerals that are expected to be present including copper, zinc, iron cobalt, manganese, and fluoride.
The function of each trace mineral varies and they play huge role in the growth and development process.
For proper functioning of human body, only small quantities of these minerals are required. Among 21 minerals, iron is found in huge quantities.
Certain foods are rich sources of these minerals. However, deficiency or excess of these minerals can lead to serious health issues.
If you need to learn more about trace minerals click here:
https://brainly.com/question/13023329
#SPJ4
1. Native copper is a mixture of two isotopes. Copper-63 contributes 69.17% of the atoms of copper and copper-65 the remaining 30.83%. What is the average atomic mass?
Answers
Answer: 63.6166 amu (atomic mass units)
Explanation: When an element X is written in the form of X-N, N will the atomic mass of that element. So we have copper with a mass of 63 and 65. We are given percentages of each one. 69.17/100 of the copper atoms will be of weight 63 while 30.83/100 will be of weight 65. Keep in mind that amu does not actually mean weight but I'm just using it as a tool to help explain the general concept. Assume you have 100 copper atoms, and you need to find the average atomic mass for them, if you do this you will solve the problem. Due to the fractions, 69.17 of them will be 63 amu and the rest will be 65. So do 69.17*63+65*30.83 and divide by one hundred. By doing this you will get 63.6166. If my answer doesn't make sense, search up how to calculate weighted averages
Hope this helps
why does salt water have more cohesion than tap water
Answers
Answer:
Cohesion exists because of the polarity of water. The water has a dipole that causes it to act like a magnet, attracting other water molecules to it. ... The salt water has a much lower cohesion than plain water so it's attractive forces are less than plain water.
Match these items.
No links please!
Definitions:
1. Built the first American A.C. generator.
2. Found a relationship between electricity and magnetism.
3. Associated with gas and oil.
4. First workable electric light bulb.
5. Developed the equations E=mc^2
6. Provides majority of the energy in the United States.
7. Splitting heavy atoms
8. Produces plutonium
9. Powered by falling water or stream pressure
10. Thin silicon slices
11. Using heat from the earth
Term names:
a. Oersted
b. Geothermal
c. Edison
d. Einstein
e. Salt dome
f. Turbine
g. Fission
h. Westinghouse
i. Solar cells
j. Gas and oil
k. Breeder
Answers
\(\huge{\mathbb{\tt { QUESTION↓}}}\)
1. Built the first American A.C. generator.
2. Found a relationship between electricity and magnetism.
3. Associated with gas and oil.
4. First workable electric light bulb.
5. Developed the equations E=mc^2
6. Provides majority of the energy in the United States.
7. Splitting heavy atoms
8. Produces plutonium
9. Powered by falling water or stream pressure
10. Thin silicon slices
11. Using heat from the earth
\(\huge{\mathbb{\tt {ANSWER↓}}}\)
\(\color{black}{\tt {1.) \: h. \: Westing \: house}}\)
Built the first American A.C. generator\(\color{black}{\tt {2.) \: Oersted}}\)
Hans Christian Oersted discovered the relationship between electricity and magnetism in 1820.\(\color{black}{\tt {3.) \: j. Gas \: and \: oil}}\)
Associated gas refers to the natural gas found in association with oil within the reservoir. There are also reservoirs that contain only natural gas and no oil, this gas is termed non-associated gas.\(\color{black}{\tt {4.) \: c.Edison}}\)
By January 1879, at his laboratory in Menlo Park, New Jersey, Edison had built his first high resistance, incandescent electric light. It worked by passing electricity through a thin platinum filament in the glass vacuum bulb, which delayed the filament from melting. Still, the lamp only burned for a few short hours.\(\color{black}{\tt { 5.) \: d. Einstein}}\)
E = mc2, equation in German-born physicist Albert Einstein's theory of special relativity that expresses the fact that mass and energy are the same physical entity and can be changed into each other.\(\color{black}{\tt {6.)i.solar cells}}\)
Energy Sources in the United StatesPetroleum (crude oil and natural gas plant liquids): 28% Coal: 17.8% Renewable energy: 12.7% Nuclear electric power: 9.6%"\(\color{black}{\tt { 7.) \: g. fission}}\)
Nuclear fission is a process by which the nucleus of an atom is split into two or more smaller nuclei, known as fission products. The fission of heavy elements is an exothermic reaction, and huge amounts of energy are released in the process.\(\color{black}{\tt { 8.) Sorry \: but \: I \: don't \: know \: the \: answer}}\)
\(\color{black}{\tt { 9.) Sorry \: but \: i \: don't \: know \: the \: answer}}\)
\(\color{black}{\tt { 10. ) \: Sorry \: \: But \: I \: don't \: know \: the \: answer }} \)
\(\color{black}{\tt {11.) \: b. Geothermal}}\)
Geothermal/GSHP systems take advantage of nature's underground temperatures by exchanging heat with the earth using an underground network of water (or refrigerant) filled pipes. In the winter, the liquid pulls heat from the ground and transfers it to the house through an exchanger.#CarryOnLearning
#LetsEnjoyTheSummer
→XxKim02xX
How much volume of butanol should be combined with 30. 0 mL of ethanol to make a 5. 88% volume percent solution of ethanol in
butanol?
Answers
We should combine 480.2 mL of butanol with 30.0 mL of ethanol to make a 5.88% volume percent solution of ethanol in butanol.
To find the volume of butanol needed to make a 5.88% volume percent solution of ethanol in butanol, follow these steps:
Step 1: Identify the given information.
- Volume of ethanol (V₁) = 30.0 mL
- Percent volume of ethanol in the solution (P) = 5.88%
Step 2: Express the volume percent as a decimal.
P = 5.88% ÷ 100 = 0.0588
Step 3: Write the formula for volume percent.
Volume percent (P) = (Volume of ethanol (V₁)) ÷ (Total volume (V_total))
Step 4: Rearrange the formula to find the total volume (V_total).
V_total = V₁ ÷ P = 30.0 mL ÷ 0.0588 ≈ 510.2 mL
Step 5: Calculate the volume of butanol (V₂).
Volume of butanol (V₂) = Total volume (V_total) - Volume of ethanol (V₁) = 510.2 mL - 30.0 mL = 480.2 mL
So, we should combine 480.2 mL of butanol with 30.0 mL of ethanol to make a 5.88% volume percent solution of ethanol in butanol.
To know more about volume visit :-
https://brainly.com/question/14197390
#SPJ11
What type of redox reactions are the following two problems fe + mgbr2 -> febr3 + mg ca(oh)2 + mgso4 -> caso4 + mg(oh)2
Answers
The first reaction is a redox reaction involving a single displacement reaction, while the second reaction is a precipitation reaction.
In the first reaction, Fe (iron) displaces Mg (magnesium) from its compound,\(MgBr2\). This indicates a transfer of electrons from Fe to Mg, resulting in the reduction of Mg and the oxidation of Fe. Therefore, it is a single displacement or substitution reaction with a redox component.
In the second reaction, \(Ca(OH)2 and MgSO4 react to form CaSO4 and Mg(OH)2.\) This reaction does not involve a transfer of electrons between species. Instead, it is a precipitation reaction where two aqueous solutions react to form an insoluble solid (precipitate). In this case, \(CaSO4\)precipitates out of the solution, while\(Mg(OH)2\)remains in the solution.
Redox reactions involve the transfer of electrons, where one species is reduced (gains electrons) and another is oxidized (loses electrons). Precipitation reactions, on the other hand, involve the formation of an insoluble solid product from the reaction of two aqueous solutions.
Learn more about redox reaction
brainly.com/question/28300253
#SPJ11
the yeastengages in photosynthesis, which produces oxygen gas, bio, quizletquizlet
Answers
The statement “the yeast engages in photosynthesis, which produces oxygen gas” is incorrect. Yeast cells do not engage in photosynthesis to produce oxygen gas.
What is photosynthesis?Photosynthesis is the process by which green plants use sunlight, carbon dioxide, and water to produce glucose (a simple sugar), which serves as food for the plant.
Oxygen is also produced during photosynthesis.Yeast:Yeast is a unicellular fungus that converts sugar into carbon dioxide and alcohol. Yeast cells respire aerobically in the presence of oxygen and anaerobically in the absence of oxygen.
However, yeast cells do not perform photosynthesis. They do not have chloroplasts or pigments that are required for photosynthesis. Therefore, yeast cells cannot produce oxygen gas.Bio quizlet:Quizlet is an online learning platform that allows users to create and share flashcards, study guides, and quizzes.
It is a great resource for studying biology and other subjects. Students can create their own study materials or use existing ones created by other users. Quizlet provides an engaging and interactive way to learn and retain information.
To know more about photosynthesis :
https://brainly.com/question/20527415
#SPJ11
1. How many sublevels are in the following principal energy levels?
a. n=1
b. n=2
c. n=3
d. n=4
e, n=5
f. n=6
Answers
Explanation:
a 1 ( only "s"
b 2 ( "s" ,"p" )
c 3 ( "s" , "p" , "d")
d 4 ( "s", "p" , "d", "f")
e 5 ( "s", "p", "d", "f", "g")
f 6 ( "s", "p", "d", "f", "g", "h" )
The number of atoms of any element in the given chemical formula is the number that is written on the food of the symbol of that element. Therefore, sublevels are present in the principal energy levels.
What is atom?Atom is the smallest particle of any matter. Atom combines to form element and element combine to form molecule or compound.
Atom consists of electron, proton and neutron. The total mass of atom is inside the nucleus. Inside the nucleus proton and neutron is there. So calculate mass of an atom, total mass of all protons is added to the total mass of neutron. Electrons revolve around the nucleus.
principal energy levels sublevels
1 s
2 s,p
3 s,p,d
4 s,p,d,f
5 s,p,d,f,g
6 s,p,d,f,g,h
Therefore, sublevels are present in the principal energy levels.
Learn more about atoms, here:
https://brainly.com/question/13518322
#SPJ2
A 57.9 gram mass has a volume of 3 cm 3. What is the density of the mass?
Answers
Answer:
The answer is 19.3 g/cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume}\\\)
From the question
mass = 57.9 g
volume = 3 cm³
We have
\(density = \frac{57.9}{3} \\ \)
We have the final answer as
19.3 g/cm³Hope this helps you
Can anyone please suggest me a good and easy project topic for physics and chemistry. For class nine or ten students.Please please please help me.
Answers
while working in the lab, a student prepares a solution by mixing nacl and h2o in a flask. after adding the salt and mixing, the student notices that there is some undissolved solid on the bottom of the flask. this is an indication that the solution is:
Answers
A saturated solution this is an indication that the solution.
A saturated solution is a solution which contains the maximum amount of solute that can be dissolved in a given amount of solvent. If there is still undissolved solid on the bottom of the flask after the student has mixed the NaCl and H2O, then this indicates that the solution is saturated, as all of the solute that can be dissolved has been dissolved.A very saturated solution is created by repeatedly adding solute to a solution up until a point when the solute manifests as a solid precipitate or crystals.
learn more about saturated solution Refer:brainly.com/question/1851822
#SPJ4
complete question:While working in the lab, a student prepares a solution by mixing NaCl and H2O in a flask. After adding the salt and mixing, the student notices that there is some undissolved solid on the bottom of the flask. This is an indication that the solution is:
A.a saturated solution.
B.a concentrated solution.
C.an unsaturated solution.
D.a supersaturated solution.
Revise this statement to make it true:
Running water makes rock edges very sharp and pointy.
NEED HELP NOW PLEASE!! WHOEVER ANSWERS FIRST GETS BRAINIEST!!! No links or I’m reporting your answer.
Answers
Answer:
Running water makes rock edges very dull because of erosion.
Explanation:
hope this helps ^_^
5. when 500.0 g of water is decomposed by electrolysis and the yield of hydrogen is only 75.3%, how much hydrogen chloride can be made if the yield of hydrogen chloride in the second reaction is 69.8%? oxygen and chlorine are in excess.
Answers
If when 500.0 g of water is decomposed by electrolysis and the yield of hydrogen is only 75.3%. The amount of hydrogen chloride that can be made if the yield of hydrogen chloride in the second reaction is 69.8% is: 1,063g.
How to find the hydrogen chlorideNumber of moles of water:
nH2O=mH2O/MMH2O
nH2O=500.0g/18.0g/mol
nH2O=27.76mol
Number of moles of hydrogen gas:
nH2=nH2O×1
nH2=27.76mol×1
nH2=27.76mol
Theoretical yield of hydrogen gas:
nH2=mH2/MMH2
27.76 mol=mH2/2.016g/mol
mH2=27.76mol×2.016g/mol
mH2=55.96g
Actual yield of hydrogen gas:
Percent yield=Actual yield/Theoretical yield×100%
75.3%=Actual yield55.96g×100%
0.753=Actual yield55.96g
Actual yield=0.753×55.96g
Actual yield=42.1g
Number of moles of actual yield:
nH2=mH2/MMH2
nH2=42.1g/2.016 g/mol
nH2=20.9mol
Number of moles of hydrogen chloride:
nHCl=nH2×2
nHCl=20.9mol×2
nHCl=41.8mol
Theoretical yield of hydrogen chloride:
nHCl=mHCl/MMHCl
41.8mol=mHCl/36.46g/mol
mHCl=41.8mol×36.46g/mol
mHCl=1,524g
Actual yield of hydrogen chloride:
Percent yield=Actual yield/Theoretical yield×100%
69.8%=Actual yield/1,524 g×100%
0.698=Actual yield/1,524g
Actual yield=0.698×1,524g
Actual yield=1,063g
Therefore the hydrogen chloride that can be made is 1,063g.
Learn more about hydrogen chloride here:https://brainly.com/question/20728408
#SPJ1
solve the ivp d2ydt2 6dydt 34y=0,y(0)=0,y′(0)=−8 the laplace transform of the solutions is l{y}
Answers
the Laplace transform of the solution to the given initial value problem is Y(s) = (-4/17) / (s + 3 + 5i) + (36/17) / (s + 3 - 5i)
What is Laplace Transformation?
Laplace transform can be solved using the definition, properties, and techniques of Laplace transforms.
Laplace transform is a mathematical tool used to solve linear differential equations. The Laplace transform is defined as the integral of a function multiplied by an exponential function.
To solve the initial value problem (IVP) with the differential equation d²y/dt² + 6dy/dt + 34y = 0, and the initial conditions y(0) = 0 and y'(0) = -8, we can use the Laplace transform.
Taking the Laplace transform of the given differential equation, we have:
s²Y(s) - sy(0) - y'(0) + 6sY(s) - 6y(0) + 34Y(s) = 0
Applying the initial conditions, we substitute y(0) = 0 and y'(0) = -8:
s²Y(s) - 8s + 6sY(s) + 34Y(s) = 0
Rearranging the terms and factoring out Y(s), we get:
Y(s) (s² + 6s + 34) = 8s
Dividing both sides by (s² + 6s + 34), we find:
Y(s) = 8s / (s² + 6s + 34)
Now, we need to decompose the denominator into its quadratic factors:
s² + 6s + 34 = (s + 3 + 5i)(s + 3 - 5i)
Using partial fraction decomposition, we can express Y(s) as:
Y(s) = A / (s + 3 + 5i) + B / (s + 3 - 5i)
Multiplying through by (s + 3 + 5i)(s + 3 - 5i), we get:
8s = A(s + 3 - 5i) + B(s + 3 + 5i)
Expanding and equating the coefficients of s, we find:
8 = A + B
0 = 3B - 5A
Solving these equations simultaneously, we find A = -4/17 and B = 36/17.
Substituting these values back into the equation for Y(s), we get:
Y(s) = (-4/17) / (s + 3 + 5i) + (36/17) / (s + 3 - 5i)
Now, taking the inverse Laplace transform, we can find the solution y(t):
y(t) = L⁻¹{Y(s)}
The inverse Laplace transform of each term can be found using the table of Laplace transforms or by using software. The solution y(t) will involve a combination of exponential functions and trigonometric functions.
Therefore, the Laplace transform of the solution to the given initial value problem is Y(s) = (-4/17) / (s + 3 + 5i) + (36/17) / (s + 3 - 5i), and the inverse Laplace transform of Y(s) will give us the solution y(t) to the initial value problem.
To learn more about Laplace Transform from the given link
https://brainly.com/question/30402015
#SPJ4
describe the essential practical details for the preparation of
pure magnesium chloride
Answers
Start with high-quality chemicals: Ensure that the magnesium oxide or magnesium hydroxide and hydrochloric acid used in the reaction are of high purity and quality.
Prepare the reaction mixture: Add a calculated amount of magnesium oxide or magnesium hydroxide to the hydrochloric acid in a reaction vessel. The reaction is exothermic, so it is important to add the magnesium oxide or magnesium hydroxide slowly to avoid overheating.
Stir the mixture: The reaction mixture should be stirred continuously using a magnetic stirrer to ensure uniform mixing and prevent the formation of lumps.
Heat the mixture: The reaction mixture should be heated using a heating mantle or hot plate to speed up the reaction. The temperature should be maintained below 100°C to prevent the formation of magnesium oxychloride.
Monitor the progress of the reaction: The reaction is complete when all the magnesium oxide or magnesium hydroxide has dissolved, and the solution is clear.
Filter the solution: The solution is then filtered to remove any insoluble impurities or byproducts.
Concentrate and crystallize the solution: The solution is concentrated by evaporation to increase the concentration of magnesium chloride. The solution is then allowed to cool slowly to room temperature to allow the formation of pure magnesium chloride crystals.
Dry and store the magnesium chloride: The crystals are then filtered, washed with cold water, and dried in an oven. The pure magnesium chloride can then be stored in airtight containers for future use.
Overall, the preparation of pure magnesium chloride involves careful attention to detail, including the use of high-quality chemicals, controlled heating and stirring, and monitoring the progress of the reaction to ensure the production of high-quality crystals.
What type of reaction is this?
N2 + 3H2
2NH3
Answers
What do l talk about on my timelines?
Answers
Timelines are intended to give a general perspective of a series of historical events. Although they don't go into great length, as necessary, connections to photographs, information, and events may be added.
What are Timelines?History textbooks and biographies frequently employ timelines to illustrate what occurred over a specific time period or to a specific person, beginning with the earliest event and going ahead in time. For instance, your own personal chronology can start with your birth.
Timelines are meant to provide a broad overview of a number of historical events.
There may be connections to images, facts, and events, albeit they don't go into great detail unless essential.
Thus, this way, one can have their timeline.
For more details regarding timeline, visit:
https://brainly.com/question/20838835
#SPJ1
Why do the protons identify the element and not the electrons or the
neutrons?
Answers
Lattice energy is an estimate of the bond
conductivity
group
length
strength
Answers
Answer:
Lattice energy is an estimate of the bond strength.
In Experiment 2 a gas is produced at the negative electrode.
Name the gas produced at the negative electrode.
Answers
In Experiment 2, the gas produced at the negative electrode is typically hydrogen (H2).
\(\huge{\mathfrak{\colorbox{black}{\textcolor{lime}{I\:hope\:this\:helps\:!\:\:}}}}\)
♥️ \(\large{\underline{\textcolor{red}{\mathcal{SUMIT\:\:ROY\:\:(:\:\:}}}}\)
Why are sodium and chlorine the largest dissolved components in ocean water? What is the most abundant dissolved gas in ocean water?
Answers
Sodium (Na) and chlorine (Cl) are the largest dissolved components in ocean water due to the abundance of sodium and chloride ions in the Earth's crust and the continuous input of these elements into the oceans through various processes. Sodium is one of the most common elements in the Earth's crust, and chlorine is widely distributed in rocks, minerals, and salts.
Over millions of years, weathering of rocks, volcanic activity, and erosion release these elements into rivers and ultimately into the oceans. The combination of sodium and chlorine ions results in the formation of sodium chloride, which is commonly known as table salt and contributes to the salinity of seawater.
The most abundant dissolved gas in ocean water is carbon dioxide (CO2). Carbon dioxide dissolves in the surface waters of the ocean through gas exchange with the atmosphere. It plays a crucial role in regulating the pH of seawater and is an essential component of the carbon cycle. Carbon dioxide is involved in various biological and chemical processes in the ocean, including photosynthesis by marine plants and the formation of calcium carbonate shells by marine organisms. Additionally, the increase in atmospheric carbon dioxide due to human activities has led to ocean acidification, which is a significant concern for marine ecosystems.
To know more about Sodium (Na) and chlorine (Cl), click here, https://brainly.com/question/5319005
#SPJ11
Due to the fact that they combine to form the ionic compound sodium chloride (NaCl), also known as salt, sodium (Na) and chlorine (Cl) are the two most abundant dissolved elements in ocean water.
Thus, Salts are among the many dissolved compounds that water from rivers and streams transports into the ocean.
In particular, sodium and chloride ions have accumulated in the ocean throughout time, leading to the high concentration of these elements in seawater. Magnesium, calcium, potassium, and sulphate ions are among the other dissolved substances in ocean water.
Oxygen is the dissolved gas that is most prevalent in ocean water.
Thus, Due to the fact that they combine to form the ionic compound sodium chloride (NaCl), also known as salt, sodium (Na) and chlorine (Cl) are the two most abundant dissolved elements in ocean water.
Learn more about Ocean, refer to the link:
https://brainly.com/question/12738467
#SPJ4
assume that heat in the amount of 100 kj is transferred from a cold reservoir at 600 k to a hot reservoir at 1250 k contrary to the clausius statement of the second law. what is the total entropy change?
Answers
total entropy change:
S total = S hot + S cold = 0.0615kJ/k
which is violation of Clausius inequality of increase of entropy principle S total >= 0
What is entropy ?
Entropy is the measurement of the amount of thermal energy per unit of temperature in a system that cannot be used for productive work. Entropy is a measure of a system's molecular disorder or unpredictability since work is produced by organized molecular motion. Entropy theory offers profound understanding of the direction of spontaneous change for many commonplace events.
Entropy is a concept that gives mathematics a way to express the intuitive understanding of which operations are impossibly impossible even though they wouldn't go against the fundamental tenet of energy conservation.
T cold = 600k
T hot = 950k
Q=100kj
Shot=-Q/T hot = -0.1052kJ/k
S cold=Q/Tcold = 0.1667kJ/k
total entropy change:
S total = Shot + S cold = 0.0615kJ/k
which is violation of Clausius inequality of increase of entropy principle S total>=0
To know more about entropy from the link
https://brainly.com/question/6364271
#SPJ4
How much Glucose should be consumed if we want to produce 2.3 moles of Carbon Dioxide (CO₂)?
Answers
In order to produce 2.3 moles of carbon dioxide (CO₂), you would need to consume 7.9 moles of glucose. This is because in the chemical equation for respiration, glucose reacts with oxygen to form carbon dioxide and water: C6H12O6 + 6O2 → 6CO2 + 6H2O.