evidence to show how oobleck behaves the way it does
Answers
Explanation:
Oobleck is a non-Newtonian fluid, which means it doesn't behave like typical liquids such as water or oil. Instead, it behaves like a mixture of a liquid and a solid, depending on how much force is applied. When oobleck is left alone, it behaves like a liquid and flows like a viscous fluid. However, when force is applied to it, such as squeezing or hitting it, it becomes solid and resists the force.
One way to see this behavior is by conducting the famous "oobleck dance" experiment. If oobleck is placed on a speaker and vibrated with sound waves, it will start to behave like a solid and form interesting patterns. The vibration causes the cornstarch particles to move closer together, forming a more solid structure. When the vibrations stop, the oobleck returns to its liquid state.
Another experiment involves making oobleck and slowly stirring it. As the oobleck is stirred, it becomes more liquid-like and easier to stir. However, if the stirring is done too quickly, the oobleck becomes more solid and resists the motion. This is due to the cornstarch particles clumping together and creating a more solid structure.
Overall, the behavior of oobleck can be explained by the properties of the cornstarch particles and how they interact with each other. Its unique properties make it a popular material for science experiments and demonstrations.
Related Questions
What is the density of a substance that mass of 13.4 g and a volume of 3.1 mL. (D=m/v)
Answers
Answer:
The answer is 269.85 lb/cu ft
Why does an "acid spill kit" often contain baking soda?
Question options:
A) Baking soda is a weaker acid, which will raise the solution's pH.
B) Baking soda will absorb the acid so that it can be swept up.
C) Baking soda is a strong base that will react violently with the acid.
D) Baking soda is a weak base and will neutralize the acid.
Answers
Baking soda neutralizes the acid so D
Baking soda is a weak base and will neutralize the acid.
Explanation:
The baking soda's chemical name is sodium hydrogen carbonate with a molecular formula of \(NaHCO_3\).It is basic in nature and neutralizes the acid.It is a weak base, actually an alkaline salt of a strong base, and weak acid.The general reaction of baking soda with acid is given as:\(NaHCO_3+HA\rightarrow NaA+H_2O+CO_2\)
(Where: HA is the general formula of an acid)
That is why the baking soda is present in the acid spill kits. So from this, we can conclude that the correct answer is option D.
Learn more about baking soda here:
brainly.com/question/4424346?referrer=searchResults
brainly.com/question/22441199
What is the∆S° of 0₂
Answers
Answer:0
Explanation: zero because it is the most stable form of oxygen in its standard state
Joelle is a manager at a construction company, and she is interested in the chemistry behind the materials they use. She has begun studying the materials used to fill walls. She knows that to keep the temperature inside a room steady the material must be a thermal insulator, and she predicts that materials should not be acidic or else they would dissolve too easily in water.
Which of these is a molecular ingredient that could be used in a wall-filling material ?
C27H36N2O10
Na6Ba6
NeNa
HCl
Answers
Answer:
a
Explanation:
A molecular ingredient that could be used in a wall-filling material is C₂₇H₃₆N₂O₁₀, is not a good thermal insulator, hence option A is correct.
The big molecule C₂₇H₃₆N₂O₁₀ is a poor thermal insulator. Since NeNa is a relatively reactive chemical, it would probably dissolve in water far too quickly. The acidic molecule HCl would dissolve far too quickly in water.
Not a good thermal insulator is C₂₇H₃₆N₂O₁₀. It is a big molecule composed of a lot of hydrogen atoms. Since hydrogen atoms are excellent heat conductors, they would be ineffective in stopping heat from passing through a material used to fill a wall.
Thus, the option (A) C₂₇H₃₆N₂O₁₀ is correct.
Learn more about thermal insulator, here:
https://brainly.com/question/23134662
#SPJ6
pls help me guys please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please please

Answers
5) B
6) C
7) B newland, law of octaves
8) A)
how are the earth and moon alike
Answers
Answer:
they both revolve or rotate around something
Explanation:
The earth revolves around the sun and the moon rotates around the earth.
Full Electron configuration (SN) show work please
Answers
Answer: Explanation:
[Kr] 4d¹⁰ 5s² 5p²
Help Please I'll mark Brainiest!
Write three observations that could help you identify this organism.

Answers
Secondly, the colors. As different organism come in different areas of reflections of light, it can easily be identified with this certain color and the way that the light reflects on it. This is another form of visual appearance, and important factor in identification.
Lastly, the pattern. As located on the back, the spikes/thread-looking things sticking out plays a huge factor in finding the organism name and/or identifying it. Every organism has a different pattern, humans being a huge one, all different
Observational studies of organisms is significant to our lives, and it’s greatly helped progress our sciences and society.
Answer:
the unidentified animal looked like a slug, it looked like it lives or can be in water, the body of the unidentified animal is purple, it seems to have an orange spikes coming out of it's backs.
what is the percent by mass of oxygen in magnesium oxide, mgo?
Answers
Magnesium Oxide is a compound of Magnesium and Oxygen. As a result, it is important to determine the percentage by mass of oxygen in Magnesium Oxide (MgO). Hence, the percentage by mass of oxygen in Magnesium Oxide (MgO) is 40%.
The mass of a compound is determined by adding the mass of its constituent elements. Magnesium has a mass of 24, while Oxygen has a mass of 16. Thus, the mass of Magnesium Oxide (MgO) is 24+16= 40.00 g/mol. Therefore, the mass of oxygen in 1 mole of MgO is 16.00 g.The percentage by mass of oxygen in Magnesium Oxide can be calculated as follows:% by mass of Oxygen = mass of Oxygen / mass of Magnesium oxide × 100The mass of oxygen in 1 mole of MgO is 16.00 g. Thus,% by mass of Oxygen = (16.00/40.00) × 100% by mass of Oxygen = 40%Hence, the percentage by mass of oxygen in Magnesium Oxide (MgO) is 40%.
To Know more about Magnesium Oxide visit:
brainly.com/question/29252085
#SPJ11
Describe the unique properties of water and how they affect the following:
phase changes
physical properties
This is my answer. Is it ok? Do you have suggestions to make it better?
Unique properties of water are polarity, solvency, cohesion, adhesion, high boiling point, density and the ability to dissolve other substances. The three phases of water are solid (frozen), liquid and gas and are changed by temperature. Water molecules don't change between the phases, the molecules just interact differently to make the change.
Answers
Unique properties of water
Water molecules have a bent overall structure, partial positive charges on the hydrogens, partial negative charges on the oxygen, and are polar. This is due to the fact that oxygen is more electronegative than hydrogen, making it more effective at drawing electrons. Excellent solvents include water.
Temperature alters the three states of water, which are solid (frozen), liquid, and gas. Although the interactions of the water molecules change, the molecules themselves do not alter between the phases.
Water quality's physical characteristics
Color: Polluted water may be coloured; pure water is colourless.
Turbidity: Clear, light-unabsorbing water is the opposite of pure water.
Taste and odour: Pure water never has a taste or an odour.
How many distinct characteristics does water have?Given their polar nature, water molecules form hydrogen bonds. This gives water its distinctive properties, including polarity, solvency, cohesion, adhesion, high specific heat, and the capacity to act as a buffer. A solute that has dissolved in a solvent creates a homogeneous mixture known as a solution.
To know more about unique properties of water visit:
https://brainly.com/question/19045958
#SPJ1
Please help fasts thanks
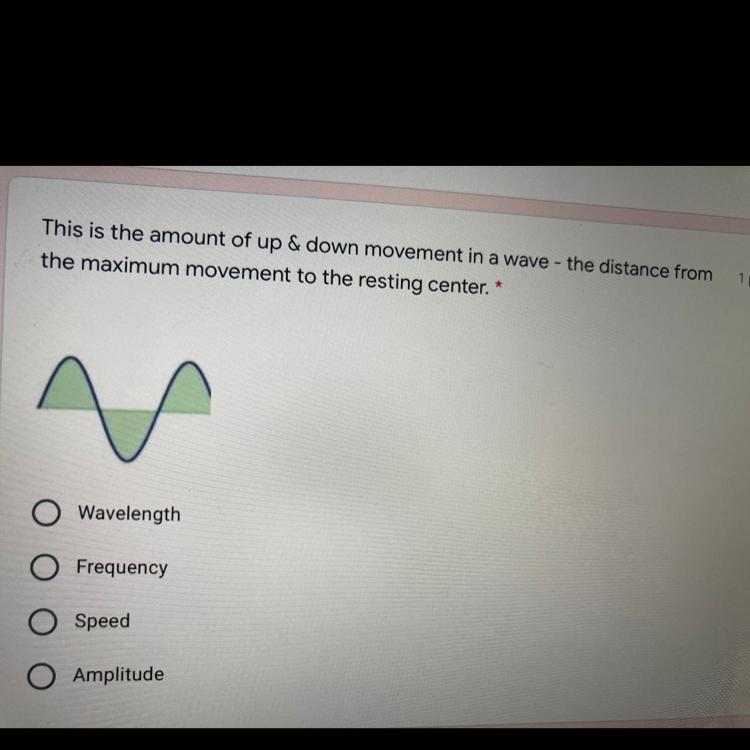
Answers
Answer:
amplitude
Explanation:
The amplitude of a wave is the distance from the centre line (or the still position) to the top of a crest or to the bottom of a trough
Answer:
I think it is wave length
Explanation:
yeah pretty sure
in constructing simple galvanic cells why it is important to use two different metals as electrodes? How can cells, madwe i this way produce electricity?explain.
Answers
This potential difference allows for the transfer of electrons from the metal with a higher energy level to the metal with a lower energy level, resulting in the production of electricity.
The process of producing electricity in a galvanic cell involves the flow of electrons from the anode (the metal with a higher energy level) to the cathode (the metal with a lower energy level) through a conductive medium (such as an electrolyte). This flow of electrons creates an electrical current that can be harnessed to power various devices.
The specific metals used in a galvanic cell will determine the potential difference and the amount of electricity that can be produced. For example, a cell constructed with zinc and copper electrodes can produce a voltage of around 1.1 volts, while a cell constructed with magnesium and silver electrodes can produce a voltage of around 2.6 volts.
To know more about electricity visit:-
https://brainly.com/question/31668005
#SPJ11
/Why does an atom want all of it’s orbitals filled
Why does it want to be in a stable state
Answers
What is the mass of 4 moles of CO₂?
Answers
The first thing we need to do is calculate the mass of one mole of carbon dioxide, and we get:
(Mass of Carbon) + (Mass of 2 Oxygen atoms) = Molar mass
Mass = 12 + (2 × 16) = 44gTherefore, carbon dioxide has a molar mass of 44 grams, which is the mass of the gas
In order to determine the mass of 4 molecules of carbon dioxide, we need to multiply it by 4 × 44 = 176g.
Hope this helps :)
\({ \qquad\qquad\huge\underline{{\sf Answer}}} \)
Here we go ~
lets calculate Molar mass of \({\sf CO_2} \) :
\(\qquad \sf \dashrightarrow \: Molar \: \: mass \: \: of \: \: C O _2 = 12 + 2(16)\)
[ Molar mass of\({\sf \: CO_2 } \)= Molar mass of Carbon + 2×( Molar mass of Oxygen )]
\(\qquad \sf \dashrightarrow \: Molar \: \: mass \: \: of \: \: C O _2 = 12 + 32\)
\(\qquad \sf \dashrightarrow \: Molar \: \: mass \: \: of \: \: C O _2 = 44 \: g\)
[ i.e 1 mole of \({\sf CO_2} \) weights 44 grams ]
So, by unitary method :
\(\qquad \sf \dashrightarrow \: 1 \: \: mole \: \: CO_2 = 44 \: \: g\)
\(\qquad \sf \dashrightarrow \: 4 \: \: moles \: \: CO_2 = (44\times 4) \: \: g\)
\(\qquad \sf \dashrightarrow \: 4 \: \: moles \: \: CO_2 = 176 \: \: g\)
Therefore, 4 moles of CO2 weights 176 grams
How many molecules does 85.0 g HCl contain?
A. 2.04 x 10^24 molecules
B. 1.98 x 10^24 molecules
C. 1.40 x 10^24 molecules
D. 2.54 x 10^24 molecules
Answers
Moles = 85/(1+35.5)
Moles = 2.33
Multiply by agrovados number (6.022 * 10^23) to get
1.401917808219x10^24 molecules
C is the answer
Hope this helped :)
Answer: C. 1.40×10^24
Explanation:
To do this, we must convert this from grams to moles, then from moles to molecules. For the first step, you will need to find the molar mass of HCl on the periodic table, which is 36.461gHCl.
\(85.0gHCl*\frac{1molHCl}{36.461gHCl} =2.33molHCl\)
Now that we have the moles, we must convert that to molecules by using Avogadro's number.
\(2.33molHCl*\frac{6.022*10^{24} }{1molHCl} =1.40*10^{24}\)
Now we have our answer. The best answer choice here is C. 1.40×10²⁴.
I hope this helps! Pls mark brainliest!! :)
PLEASE HELP ME OUT. I AM REALLY LOST AND I JUST NEED THIS LAST QUESTION I WILL BE GRATEFUL AND WILL GIVE BRANLIEST!
PLEASE HELP ME OUT. I AM REALLY LOST AND I JUST NEED THIS LAST QUESTION I WILL BE GRATEFUL AND WILL GIVE BRANLIEST!
PLEASE HELP ME OUT. I AM REALLY LOST AND I JUST NEED THIS LAST QUESTION I WILL BE GRATEFUL AND WILL GIVE BRANLIEST!

Answers
Explanation:
—COOH and —NH₂
Carboxylic Acid and Amino
587. mL of 0.00531 M NaI (aq) is combined with 840. mL of 0.00536 M Pb(NO3)2 (aq). Determine if a precipitate will form given that the Ksp of Pbl2 is 1.40x10-8.
a. Precipitation will not occur because Qsp > Ksp
b. Precipitation will occur because Qsp > Ksp
c. Precipitation will occur because Qsp = Ksp
d. Precipitation will not occur because Qsp < Ksp
e. Precipitation will occur because Qsp < Ksp
Answers
The formation of a precipitate is possible when the product of the ionic concentrations exceeds the Ksp value. Qis is the reaction quotient, which is the ionic product (IP) in a solution.
To determine whether a precipitate will occur, the reaction quotient (Qis) must be compared to the solubility product constant (Kip). The correct option is (d) Precipitation will not occur because Qis < Kip. The calculations are provided solution below; Qis = [Pb2+] [I–]2Moles of NaI = 0.587 L × 0.00531 mol/L = 0.00313 mol Moles of Pb(NO3)2 = 0.840 L × 0.00536 mol/L = 0.00451 mol[Pb2+] = 0.00451 mol / (0.587 L + 0.840 L) = 0.00327 M[I–] = 0.00313 mol / (0.587 L + 0.840 L) = 0.00226 MQsp = (0.00327 M) × (0.00226 M)2 = 1.72 × 10–8 Kip = 1.4 × 10–8As Qsp is less than Ksp, a precipitate will not form. Therefore, the correct option is (d) Precipitation will not occur because Qis < Ksp.
learn more about solution here.
https://brainly.com/question/1616939
#SPJ11
Let AC(q) and MC(a) be the average cost function and the marginal cost function respectively. Which of the following conditions guarantee that AC(q) has a local minimum at q = 27?
Answers
To determine the conditions that guarantee a local minimum of the average cost function (AC(q)) at q = 27, we need to consider the relationship between AC(q) and MC(a) at that point.
To determine the conditions that guarantee a local minimum of the average cost function (AC(q)) at q = 27, we need to consider the relationship between AC(q) and MC(a) at that point.
Here are the possible conditions:
1. AC(q) = MC(a) at q = 27: If the average cost (AC) is equal to the marginal cost (MC) at q = 27, it suggests that the average cost is not changing at that point. This condition could indicate a local minimum for AC(q) at q = 27.
2. AC(q) < MC(a) for q < 27 and AC(q) > MC(a) for q > 27: If the average cost is lower than the marginal cost for q values less than 27 and higher than the marginal cost for q values greater than 27, it suggests that AC(q) is decreasing before q = 27 and increasing after q = 27. This condition could indicate a local minimum for AC(q) at q = 27.
To know more about average cost function follow the link:
https://brainly.com/question/29360248
#SPJ4
Task 3: Below are data you collected from a
reservoir. Complete tasks 3a and 3b.
Hardap Dam water parameters for different depths
taken:
Depth
Oxygen
pH
Temperature
Surface
7.5
7.9
Answers
It can be assumed that it is within the acceptable range for domestic use. Rapid temperature changes or high temperatures can also cause damage to pipes and fittings.
Task 3a: Discuss the significance of the measured water parameters collected from Hardap Dam for aquatic life.Water parameters are chemical, biological and physical characteristics of the water. They are important indicators of the quality of water. Hardap Dam is a habitat for a variety of aquatic life such as fish, birds, plants and insects. The measured water parameters for different depths include oxygen, pH and temperature.The concentration of oxygen in the water is crucial to aquatic life. Oxygen is required for respiration by aquatic animals. The surface oxygen concentration of 7.5 mg/L measured in Hardap Dam is adequate for most aquatic life. However, some fish species require higher concentrations of dissolved oxygen to survive. As water depth increases, the oxygen concentration decreases.
This can be seen in the decreasing oxygen concentration at depths below the surface. Low oxygen concentration can lead to suffocation of aquatic life, changes in species composition and nutrient cycling.PH is a measure of the acidity or alkalinity of water. Aquatic life requires a pH range of 6.5-9.0 to survive. The pH of 7.9 measured at the surface of Hardap Dam indicates that the water is slightly alkaline. The pH values for the deeper water layers were not provided but it can be assumed that they are likely to be similar. Extreme pH values can lead to stress and death of aquatic organisms.Temperature is an important parameter that influences the metabolic rates of aquatic organisms. Temperature affects the solubility of oxygen and other gases in water. It also determines the rate of biochemical reactions in organisms. The temperature of the water at the surface of Hardap Dam was not provided.
However, it can be assumed that it is within the range of tolerance for most aquatic organisms.
As water depth increases, temperature decreases. Rapid temperature changes or high temperatures can cause stress and death in aquatic organisms. Task 3b: Evaluate the significance of the measured water parameters in relation to the use of Hardap Dam water for domestic purposes.Water is a critical resource for human survival. Hardap Dam supplies water to communities for domestic purposes. The water quality is important to prevent the spread of diseases and illness. The measured water parameters for different depths of the dam include oxygen, pH and temperature.
These parameters affect the suitability of the water for domestic use.Oxygen concentration in the water is important for the removal of pollutants and odours. The surface oxygen concentration of 7.5 mg/L measured in Hardap Dam is adequate for this purpose. As the water depth increases, oxygen concentration decreases. Low oxygen concentration can lead to unpleasant tastes and odours in the water.PH is important for the taste and aesthetics of water. The pH of 7.9 measured at the surface of Hardap Dam is within the acceptable range for domestic use. Extreme pH values can cause water to taste bitter or metallic.
Changes in pH can also affect the corrosion of pipes and fittings.Temperature can affect the growth of microorganisms in the water. High temperatures can promote the growth of harmful bacteria such as E. coli. The temperature of the water at the surface of Hardap Dam was not provided. However, it can be assumed that it is within the acceptable range for domestic use. Rapid temperature changes or high temperatures can also cause damage to pipes and fittings.
Learn more about Values here,https://brainly.com/question/11546044
#SPJ11
The equilibrium concentrations were determined to be: NCI3 = 0.5 M, N2 = 0.18 M and C12 = 0.25 M. What is the Kc value for this reaction?
Answers
When a chemical process reaches equilibrium, the equilibrium constant (often represented by the letter K) sheds light on the interaction between the reactants and products. The value of Kc is 88.96.
The ratio of the concentration of the products to the concentration of the reactants, each raised to their respective stoichiometric coefficients, is the equilibrium constant of concentration (denoted by Kc) of a chemical process at equilibrium.
Here the reaction is:
N₂ + 3Cl₂ → 2NCl₃
Kc = [NCl₃]² / [N₂] [Cl₂]³
Kc = (0.5)² / (0.18) (0.25)³ = 88.96
To know more about equilibrium constant, visit;
https://brainly.com/question/29253884
#SPJ1
Para producir una figura de bronce se han mezclado 750 gramos de cobre y 50 gramos de estaño al medir la masa de la figura se encuentra q tiene un valor de 800 gramos
Answers
To produce a bronze figure, 750 grams of copper and 50 grams of tin have been mixed.
To produce a bronze figure, 750 grams of copper and 50 grams of tin have been mixed. When measuring the mass of the figure, it is found that it has a value of 800 grams.Bronze is an alloy formed by mixing copper and tin in certain proportions.
When a specific amount of copper and tin is mixed, the alloy's properties and color can vary. A bronze figure is produced by mixing 750 grams of copper and 50 grams of tin. Since copper is the main constituent of bronze, the bronze will be mostly copper with tin added to it.
The sum of the two metals' masses is equal to 750 + 50 = 800 grams
. Since the bronze figure weighs 800 grams, it contains all of the metals that were added.
This is because when two substances combine chemically, their masses are combined as well.So, to produce a bronze figure, 750 grams of copper and 50 grams of tin have been mixed.
For more such questions on bronze visit:
https://brainly.com/question/3244358
#SPJ8
Note the translated question is:
To produce a bronze figure, 750 grams of copper and 50 grams of tin have been mixed, when measuring the mass of the figure, it is found that it has a value of 800 grams
A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
Between Na and Na+ which has larger size
Answers
Answer:
Na+ is smaller than Na because, it has given away one electron because of which the electron shielding gets stronger due to more protons and less electrons. Whereas, Cl- is larger than Cl because it has gained an extra electron and so, the no.07/12/2010
Explanation:
Consider the reaction
2C2H6(g) + 7O2(g)4CO2(g) + 6H2O(g)
Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 2.31 moles of C2H6(g) react at standard conditions.
S°surroundings =_________ J/K
Answers
The entropy change for the surroundings is 5226.2 J/K. This means that the surroundings become more disordered and the entropy of the universe increases during this reaction.
To calculate the entropy change for the surroundings, we need to first determine the entropy change for the system, which in this case is the reaction of 2.31 moles of C2H6(g) at standard conditions.
Using standard thermodynamic data, we can write the balanced chemical equation and calculate the standard entropy change (∆S°) for the system:
C2H6(g) + 7/2O2(g) → 2CO2(g) + 3H2O(l); ∆S° = -292.1 J/K·mol
Next, we can use the equation:
∆S°surroundings = -∆H°system/T
where ∆H°system is the standard enthalpy change for the system, and T is the temperature in Kelvin. At standard conditions, ∆H°system is -1560.8 kJ/mol for this reaction. Converting the temperature of 298 K to Kelvin, we get:
T = 298 K
Plugging these values into the equation, we get:
∆S°surroundings = -(-1560.8 kJ/mol)/298 K
= 5226.2 J/K
To know something about the entropy change, click below.
https://brainly.com/question/31428398
#SPJ11
What period is the atom in?
What group is the atom in?
What is the name of this atom?
What other atom would have similar properties
to the atom?
Would this atom be malleable or brittle?

Answers
It is in group 17
It is a chlorine atom because it has 17 electrons which means the atomic number is 17
The following flow of energy takes place in one ecosystem: alga → insect → larva flatworm → fish.
Which trophic level has the most energy to pass on to the next?
A) insect larva
B) fish
C) flatworm
D) alga
Answers
Answer: D. Alga
Explanation:
What is the wavelength of the photons emitted by hydrogen atoms when they undergo n = 4 to n = 2 transitions? ___nm
In which region of the electromagnetic spectrum does this radiation occur?
a. Infrared
b. ultraviolet
c. Microwaves
d. visible
Answers
Answer: To find the wavelength of the photons emitted by hydrogen atoms when they undergo n = 4 to n = 2 transitions, we can use the Rydberg formula:
1/λ = R_H * (1/n1² - 1/n2²)
Where λ is the wavelength, R_H is the Rydberg constant for hydrogen (approximately 1.097 x 10^7 m^-1), n1 and n2 are the initial and final energy levels, respectively.
Explanation:
The formula used to determine the wavelength of light is known as the Rydberg formula. The energy of an electron changes when it transitions from one atomic orbit to another. The photon of light is produced when the electron transitions from a high-energy orbit to a lower-energy state. Additionally, the photon of light is absorbed by the atom when the electron transitions from a low energy to a higher energy state.
In this case, n1 = 2 and n2 = 4. Plugging the values into the formula, we get:
1/λ = (1.097 x 10^7) * (1/2² - 1/4²)
1/λ = (1.097 x 10^7) * (1/4 - 1/16)
1/λ = (1.097 x 10^7) * (12/64)
λ = 1 / (1.097 x 10^7 * 12/64)
λ ≈ 4.86 x 10^-7 m
Converting meters to nanometers (1 m = 1 x 10^9 nm):
λ ≈ 486 nm
The wavelength of the photons emitted by hydrogen atoms when they undergo n = 4 to n = 2 transitions is approximately 486 nm. This radiation occurs in the visible region of the electromagnetic spectrum.
Answer: The wavelength of the photons emitted by hydrogen atoms when they undergo n = 4 to n = 2 transitions is approximately 486 nm, and this radiation occurs in the visible region of the electromagnetic spectrum (option d).
To know more about Rydberg constant, visit:
https://brainly.com/question/14655295
#SPJ11
EASY 100 POINTS, WILL MARK BRAINLEST, 2 QUESTIONS, SHOW YOUR WORK OR NO POINTS

Answers
Answer:
Explanation:
1. Please provide the enthalpy info - I will work on it with the info
2.
i) Reaction a should be modified to match the number of S in equation:
2S + 2O2 -> 2SO2 deltaH = -370kJ
ii) Reaction b should be written reversely to match the reactants of SO2:
2SO2 + O2 -> 2SO3 deltaH = 256kJ
iii) Adding the equations together:
2S + 3O2 -> 2SO3
iv) Enthalpy of the combined reaction = -370+256 = -114kJ
It is negative so the reaction is exothermic.
Answer:
Explanation:
1 c. enthalpy change = enthalphies of formation of all products - enthalphies of formation of all reactants = -526.3 kcal/mol
1 d. C3H8(g) + 5 O2 (g) → 3 CO2 (g) + 4 H2O (l) ΔH = -526.3 kcal/mol
What is the wavelength of x-rays having a frequency of 4.80 x 1017 Hz?
Answers
Answer:
Refer to the attachment

What is the molar solubility of AgCl in a 0.050 M NaCl solution? The Ksp of AgCl is 1.6 x 10-10. (Assume that the contribution of [Cl-] from AgCl is negligible relative to the [Cl-] from NaCl)
Answers
The molar solubility of AgCl:
The molar solubility of AgCl in a 0.050 M NaCl solution is 8 x \(10^{-8}\) M \(Ag^{+}\)
What is solubility?
The solubility is the quantity of reagent required to saturate the solution or bring about the dissociation reaction's equilibrium.
Reaction:
The dissociation reaction of AgCl in water is:
\(AgCl\) ⇄ \(Ag^{+} + Cl^{-}\)
Each mole of AgCl that dissolves in this reaction yields 1 mole of both \(Ag^{+}\) and \(Cl^{-}\). The concentration of either the Ag or Cl ions would then be equal to the solubility.
Solubility= [\(Ag^{+}\)] = [\(Cl^{-}\)]
Calculation:
in 0.050 M NaCl, the [\(Cl^{-}\)] = 1 x \(10^{-2}\)
ksp = [\(Ag^{+}\)] x [\(Cl^{-}\)]
1.6 x \(10^{-10}\) = [\(Ag{+}\)] x ( 5 x \(10^{-2}\))
[\(Ag^{+}\)] = 5 x \(10^{+2}\) x 1.6 x \(10^{-10}\)
[\(Ag^{+}\)] = 5 x 1.6 x \(10^{-10+2}\)
[\(Ag^{+}\)] = 8 x \(10^{-8}\) M
Learn more about molar solubility here,
https://brainly.com/question/13202097
#SPJ4