Ethylene oxide (EO) is prepared by the vapor-phase oxidation of ethylene. Its main uses are in the preparation of the antifreeze ethylene glycol and in the production of poly(ethylene terephthalate), which is used to make beverage bottles and fibers. Pure EO vapor can decompose explosively:
Answers
primary applications are in the production of poly (ethylene terephthalate), which is used to make beverage bottles and fibers, as well as in the preparation of the antifreeze ethylene glycol. Pure EO vapor can explode when it decomposes: CH2=CH2(g)→CH4(g)+CO(g)
Polyethylene terephthalate glycolPETG or PET-G, also known as polyethylene terephthalate glycol, is a thermoplastic polyester with notable chemical resistance, durability, and formability for manufacturing. PET (Polyethylene Terephthalate) has been modified to form PETG, where the "G" stands for glycol, which is added at the molecular level to provide various chemical qualities. The glycol-modified PETG contains the same monomers as PET, but PETG is stronger, more durable, impact-resistant, and better suited to higher temperatures.
Learn more about Polyethylene terephthalate glycol here:-
https://brainly.com/question/19755664
#SPJ4
Related Questions
Gallium has two naturally occurring isotopes, 69Ga and 71Ga, and has an average atomic mass of 69.72 amu. Which isotope is most abundant
Answers
The most abundant isotope of gallium is 69Ga based on its higher abundance and contribution to the average atomic mass of 69.72 amu.
The most abundant isotope of gallium is determined by comparing the relative abundances of the two isotopes, 69Ga and 71Ga. Given that the average atomic mass of gallium is 69.72 amu, we set up the following equation:
(69Ga x abundance of 69Ga) + (71Ga x abundance of 71Ga) = 69.72 amu
We assume the abundance of 69Ga is x (in decimal form) and the abundance of 71Ga is (1 - x) since the sum of the abundances should be equal to 1. Plugging these values into the equation, we have:
(69Ga x) + (71Ga (1 - x)) = 69.72 amu
Simplifying the equation:
69Ga x + 71Ga - 71Ga x = 69.72 amu
2Ga - 2Ga x = 69.72 amu
2Ga (1 - x) = 69.72 amu
Dividing both sides by 2:
Ga (1 - x) = 34.86 amu
Now, we know that the atomic mass of 69Ga is less than that of 71Ga, so the abundance of 69Ga must be higher for the average atomic mass to be closer to 69.72 amu. Therefore, the most abundant isotope of gallium is 69Ga.
To know more about isotope, refer to the link :
https://brainly.com/question/27475737#
#SPJ11
a certain substance has a density of 19.3 g/cm3. what volume (in cm3) would be occupied by a 1255mg sample of this substance? report your answer to proper significant figures.
Answers
To find the volume of a 1255 mg sample of a substance with a density of 19.3 g/cm3, we need to divide the mass by the density.
The formula for calculating the volume of a substance from its mass and density is given by the equation:
Volume = Mass / Density
In this case, the mass of the sample is 1255 mg, which is equivalent to 1.255 g. The density of the substance is 19.3 g/cm3. Plugging in the values, we get:
Volume = 1.255 g / 19.3 g/cm3 = 0.0649 cm3
So, a 1255 mg sample of the substance with a density of 19.3 g/cm3 would occupy a volume of 0.0649 cm3. This answer should be reported to three significant figures, which is consistent with the precision of the mass measurement.
learn more about Volume here:
brainly.com/question/13338592
#SPJ4
how much energy is required to take a 15.0-g sample of ice at −15.0 °c to liquid water at 45.0 °c?
Answers
8.30 kJ. I hope this helps!
The energy is required to take a 15.0-g sample of ice at −15.0 °c to liquid water at 45.0 °c is 8.30kj.
What is enthalpy ?The term enthalpy is defined as the sum of the system's internal energy and the product of its pressure and volume.
Ice warming Cs = 2.09 J/g∙°C
Enthalpy of fusion ∆H = 6.02 kJ/mol
Liquid water warming Cs = 4.18 J/g∙°C
Enthalpy of vaporization ∆H = 40.7 kJ/mol
Steam Warming Cs = 2.01 J/g∙°C
Step 1: Warm ice from −15.0°C to 0°C.
q1 = 470.25 joule
Step 2: Melt ice.
q2 = 5016.66
Step 3: Warm liquid water from 0°C to 45.0°C.
q3 = 2821.5 joule
The total energy is the sum of the energy of each step.
q = q1 + q2 + q3
= 470.25 + 5016.66 + 2821.5
= 8.30kj
Thus, energy is required to take a 15.0-g sample of ice at −15.0 °c to liquid water at 45.0 °c is 8.30kj.
To learn more about the enthalpy, follow the link;
https://brainly.com/question/3393755
#SPJ2
An old refrigerator is rated at 500 W how many kilowatt hours of electric energy what does refrigerator use in 30 days assume the refrigerator is running 12 hours per day
Answers
The refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
To calculate the kilowatt-hours (kWh) of electric energy used by the refrigerator in 30 days, we need to multiply the power rating by the total running time.
Given:
Power rating of the refrigerator = 500 W
Running time per day = 12 hours
Number of days = 30
First, we need to convert the power rating from watts to kilowatts:
Power rating = 500 W / 1000 = 0.5 kW
Next, we calculate the total energy used in kilowatt-hours (kWh) over the 30-day period:
Energy used = Power rating × Running time × Number of days
Energy used = 0.5 kW × 12 hours/day × 30 days
Energy used = 180 kWh
Therefore, the refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
For more question on energy
https://brainly.com/question/29339318
#SPJ8
Which of the fallowing scientific claims about the bond in the molecular compound HF is most likely to be true?
There is a partial negative charge on the H atom.
Electrons are shared equally between the H and F atom.
The bond is extremely weak.
The bond is highly polar.
Answers
The bond is highly polar is the scientific claim about the bond in the molecular compound HF is most likely to be true.
The term polar means that there is separation of charges, the bonds will be the more polar the greater the difference in electronegativity between the bonded atoms.
In hydrogen fluoride, for example, F is more electronegative than H because its nucleus, with 9 positive charges, attracts e-shares with H more strongly than the nucleus of H, with only one positive charge.Therefore, the e- shared by covalence will be closer to F than to H and the molecule forms a permanent dipole.Therefore, we can conclude that the bond is highly polar is the scientific claim about the bond in the molecular compound HF is most likely to be true.
Learn more here: https://brainly.com/question/6205088
HELP I NEED THIS DONE BY 11:40
1. What is the force that caused the early Earth's molten materials move and flow
2.Write a sentence or two summarizing how gas and dust become planets.
Answers
2. It becomes planets because large clouds of gas and dust are called solar nebula’s. Particles collided because of gravity and larger bodies began to grow.
PLZ HELP ITS DUE IN 17 MINUTES
When water is changed from a liquid to a gas, what occurs
A. The atoms lose electrons.
B. A new compound is formed.
C. Molecules spread farther apart.
D. Hydrogen is separated from oxygen
Answers
Answer:
c
Explanation:
they spread farther apart as they lose their structure
16.
Magnesium reacts with hydrochloric acid according to the equation below.
Magnesium bertindak balas dengan asid hidroklorik mengikut persamaan berikut.
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
What is the volume of hydrogen gas produced at room condition if 0.6 g of
magnesium is added to the excess of sulphuric acid?
[Relative atomic mass : Mg = 24 : molar volume = 24 dmº mol' at room condition]
Berapakah isipadu gas hidrogen yang dihasilkan pada keadaan bilik jika 0.6 g
magnesium ditambahkan kepada asid hidroklorik berlebihan."
[Jisim atom relative:Mg = 24 : isipadu molar = 24 dm mol! pada keadaan bilik]
A 0.6 cm
B 6.0 cm
C 60.0 cm
D 600 cm
Answers
compute the fermi energy and the fermi temperature for silver. show that for t = 300 k about 0.1 percent of the free electrons in metallic silver have an energy greater than ef.
Answers
Fermi Energy: The Fermi Energy of silver is 5.51 eV.Fermi Temperature: The Fermi Temperature of silver is 8.77 × 10^4 K.
What is silver ?Silver is a precious metal element with the atomic number 47 and the chemical symbol Ag. It is a soft, white, lustrous transition metal that is highly malleable and ductile. Silver often occurs in its native form, and when found in nature is usually found combined with other metals, such as gold, lead and copper. Silver is one of the most widely used metals, both in its pure form and as alloys. It has many uses, including coins, jewellery, tableware, electronics, photography and medical applications.
At a temperature of 300 K, 0.1% of the free electrons in metallic silver will have an energy greater than the Fermi energy of 5.51 eV. This can be calculated using the Fermi-Dirac distribution, which is used to describe the probability of a particle having an energy greater than the Fermi energy. According to the Fermi-Dirac distribution, the probability of a particle having an energy greater than the Fermi energy is given by:p = 1/(1 + e^(-(E-Ef)/(kT)) ,Where E is the energy of the particle, Ef is the Fermi energy, k is Boltzmann's constant and T is the temperature.Substituting the values for Ef, k and T for silver at 300 K into the equation, we get:p = 1/(1 + e∧(-(E-5.51)/(8.62 × 10^-5)) .
To learn more about silver
https://brainly.com/question/18503331
#SPJ4
point Deshawn ran the 400 m race(in a straight line) in 2 minutes. What is his distance and displacement ? Explain your answer below in complete sentences.
Answers
His distance and displacement are the same, which was 400 m
Further explanationGiven
Distance = 400 m
time = 2 min
Required
Distance and displacement
Solution
Distance is a scalar quantity that indicates the length of the trajectory that is traveled by an object within a certain interval. Distance has no direction, only has magnitude
Can be simplified distance = totals traveled
Displacement is a vector quantity that shows changes in the position of objects in a certain interval of time. Displacement has magnitude and direction
Can be simplified displacement = distanced traveled from starting point to ending point
From the definition above shows that the displacement and the distance that he traveled have the same value (magnitude), which is equal to 400 m
The value of the two will be different if he starts and finishes at the same point, then the displacement value is zero while the distance he has traveled is still 0
Please help me with 1,2,3,4,5,6

Answers
Answer:
1a 2b 3c 4d 5c 6c that is the answer to your quesrion i believe
FILL IN THE BLANK. ______ contain(s) many ______, and ______ are instructions for producing ______.
Answers
Chromosomes contain many genes, and genes are instructions for producing proteins.
Chromosomes are thread-like objects that are found in the nucleus (core) of cells. One chromosome is made up of one protein and one DNA molecule. Different chromosome sizes can be packed together into a nucleus thanks to proteins called histones. Our chromosomes would be as tall as ours without them. The exact instructions needed by your cells to create a distinct you are provided by your chromosomes.
There should be 23 pairs of chromosomes in humans (46 total). Chromosomes are divided into one pair of sex chromosomes and 22 numbered pairs (autosomes) (X and Y). You receive a pair of chromosomes, one from each parent. The building blocks of your body are your genes. The instructions to produce proteins are provided by some genes. Your body receives instructions from proteins about the kinds of physical traits you ought to have, such as your hair and eye colour. Some genes produce RNA that performs additional functions.
Hence, genetic information is translated into protein based on need.
To know more about Genome.
https://brainly.com/question/29482089
#SPJ4
What property of a metal does the image represent
Answers
Answer:
malleable
Explanation:
The image represent in malleable property of metal.
The image possibly represents the photoelectric effect of a metal, which is when it emits electrons after being exposed to electromagnetic radiation. Metals are also characterized by physical properties such as conductivity, malleability, metallic luster, and metallic bonding.
Explanation:Based on your question, the image possibly represents the photoelectric effect, a key property of metals. This phenomenon occurs when a metal surface exposed to electromagnetic waves of a certain frequency absorbs radiation and emits electrons. These emitted electrons are called photoelectrons. Metals can also exhibit free electron model behavior, where electrons freely roam within the metal structure.
Metals possess unique physical properties like conductivity, malleability, and metallic luster. Malleability refers to the metal's ability to deform without breaking, while conductivity refers to the metal's ability to transfer heat or electricity. A metallic luster gives metals their characteristic shiny appearance.
Finally, metals are also known for their metallic bonding—a unique force that holds together the atoms within a metallic solid. Metallic bonding gives rise to many useful and varied bulk properties of metals.
Learn more about Properties of Metals here:https://brainly.com/question/33514448
#SPJ2
Which of the following can be mixed in solution with H₂CO3 to make a buffer?
OA. Na₂CO3
OB. HF
O C. NaHCO3
OD. NH3
SUBMIT
Answers
NaHCO3 is can be mixed in solution with H₂CO3 to make a buffer.
Sodium bicarbonate (IUPAC name: sodium bicarbonate, commonly known as baking soda or sodium bicarbonate) is a compound with the chemical formula NaHCO3. It is a salt composed of a sodium cation (Na+) and bicarbonate anion (HCO3-). Sodium bicarbonate is a crystalline white solid that often looks like a fine powder. It has a slightly salty, alkaline taste similar to soda carbonate (sodium carbonate). The natural mineral form is Narcolite. It is a component of sodium bicarbonate and is dissolved in many mineral springs.
Sodium bicarbonate is an antacid used to relieve heartburn and acid indigestion. Doctors may also prescribe sodium bicarbonate to reduce the acidity of blood and urine under certain conditions.
Learn more about Sodium bicarbonate here: https://brainly.com/question/20693952
#SPJ1
the proton nmr spectrum of an aromatic compound, c8h8br2, includes two methyl singlets. its proton-decoupled 13c nmr spectrum displays a total of six peaks. of the following compounds, which structure best fits these data?
Answers
The structure that best fits the given data is 1,4-dibromobenzene.
The presence of two methyl singlets in the proton NMR spectrum indicates the presence of two methyl groups in the compound. This suggests the presence of a substituent attached to the benzene ring.
The proton-decoupled 13C NMR spectrum displays six peaks, indicating the presence of six distinct carbon environments. In 1,4-dibromobenzene, there are two carbon atoms attached to the methyl groups, which gives two peaks. The benzene ring itself has four unique carbon environments, each with a different chemical shift, resulting in four additional peaks.
The structure of 1,4-dibromobenzene matches the data because it contains two methyl groups and displays a total of six peaks in the proton-decoupled 13C NMR spectrum, consistent with the given information.
To learn more about NMR spectrum, here
https://brainly.com/question/30465398
#SPJ4

Suppose the interaction between two atoms by the Lennard-Jones potential: ULJ = B/r^12 - A / r^6 where the values of A and B are known to be A = 10^-77 J x m^6 and B = 10^-134 J x m^12.
What does the Lennard-Jones potential predict for the separation r=r eq
hen the energy is at the minimum (equilibrium) value, U min. What is the u min fot this interaction at T=298 K ? What is the ratio of U min to the purely attractive van der Waals component of the interaction potential at r eq.
What is the ratio of r eq to r 0 defined by u(r 0 )=0. 4. What is the ratio of r s to r 0 , where r s is the separation where the magnitude of the (attractive adhesion) force is maximum, F max . What is the value for F max between the two atoms?
Answers
a) The Lennard-Jones potential predicts the separation r_eq at the minimum energy U_min.
b) The U_min for this interaction at T=298 K is the value obtained from the Lennard-Jones potential equation when r=r_eq.
c) The ratio of U_min to the purely attractive van der Waals component of the interaction potential at r_eq can be calculated by comparing the attractive part (-A/r^6) to the total potential energy U_min.
d) The ratio of r_eq to r_0, where u(r_0)=0.4, can be determined by finding the value of r_eq where the potential energy is equal to 0.4 times the total potential energy at r=r_0.
e) The ratio of r_s to r_0, where r_s is the separation where the magnitude of the attractive adhesion force is maximum, can be determined by finding the value of r where the derivative of the potential energy with respect to r is equal to zero.
f) The value of F_max between the two atoms can be obtained by taking the negative derivative of the potential energy equation with respect to r and evaluating it at r=r_s.
a) The Lennard-Jones potential provides information about the relationship between energy and separation between two interacting atoms.
At the minimum energy (U_min), the potential predicts the separation r_eq, which corresponds to the equilibrium distance between the atoms. This is the distance at which the energy of the system is at its lowest point.
b) To determine the value of U_min at a given temperature (T=298 K), you can substitute the equilibrium separation r_eq into the Lennard-Jones potential equation and calculate the resulting energy value.
This will give you the U_min for the interaction.
c) The Lennard-Jones potential consists of two components: an attractive component (-A/r^6) and a repulsive component (B/r^12).
The ratio of U_min to the purely attractive van der Waals component of the interaction potential at r_eq can be calculated by comparing the magnitude of the attractive component to the total potential energy at the equilibrium separation.
This ratio provides insights into the relative contribution of the attractive force to the overall potential energy at equilibrium.
d) The ratio of r_eq to r_0 can be determined by finding the value of r_eq where the potential energy is equal to 0.4 times the total potential energy at r=r_0.
In other words, you need to solve the Lennard-Jones potential equation for r_eq when the potential energy is equal to 0.4 times the potential energy at r=r_0.
e) The ratio of r_s to r_0 is obtained by finding the value of r where the magnitude of the attractive adhesion force is maximum.
This can be determined by finding the separation r where the derivative of the potential energy equation with respect to r is equal to zero.
The value of r_s represents the separation at which the attractive force between the atoms is strongest.
f) The value of F_max between the two atoms can be obtained by taking the negative derivative of the Lennard-Jones potential energy equation with respect to r and evaluating it at r=r_s.
This will give you the magnitude of the maximum attractive adhesion force between the atoms.
To know more about "Lennard-Jones potential" refer here:
https://brainly.com/question/32318368#
#SPJ11
010)
Which solution is the most concentrated?
A. 75% isopropyl alcohol
B. 7% isopropyl alcohol
C. 95% isopropyl alcohol
11)
Answers
Answer:
C
Explanation:
95 > 7
95 > 75
The answer is C.
How much heat is required to convert 12.0 g of ice at −10.0 ' C fo steam at j00.0CR 2 s
C
C
,T
2
+20
∘
C and 7 s
∘
−50
0
C. What is 13 ?
Answers
The heat required to convert 12.0 g of ice at −10.0°C to steam at 100.0°C is 3081.2 kJ. To find out the answer, we need to use the formula:
q = m[C(T2 - T1) + Lf + C(T3 - T2) + Lv + C(T4 - T3)]
where, q is the heat absorbed by the system, m is the mass of the substances specific heat capacity, C is the specific heat capacity of the substance, Latent Heat, Lf is the latent heat of fusion, Lv is the latent heat of vaporization, T1 is the initial temperature, T2 is the melting point of the substance, T3 is the boiling point of the substance, T4 is the final temperature,
Given the values, m = 12.0 gC = 4.18 J/(g °C)T1 = -10.0°C (temperature of ice)T2 = 0°C (melting point of ice)T3 = 100°C (boiling point of water)T4 = 100°C (temperature of steam)Latent heat of fusion of ice, Lf = 334 J/g, Latent heat of vaporization of water, Lv = 2260 J/g, Putting these values in the formula, we get,
q = (12.0 g)[4.18 J/(g °C)][(0°C - (-10.0°C)) + 334 J/g + 4.18 J/(g °C)[(100°C - 0°C)) + 2260 J/g + 4.18 J/(g °C)(100°C - 100°C)]q = 3081.2 J = 3.0812 kJ
Thus, the heat required to convert 12.0 g of ice at −10.0°C to steam at 100.0°C is 3081.2 kJ.
Learn more about heat: https://brainly.com/question/934320
#SPJ11
CHEMISTRY 50 POINTS!
Which of the following explains the VSEPR geometry of a water molecule?
A) It is tetrahedral because there are four bonded pairs around oxygen.
B) It is bent because there are four bonded pairs around oxygen.
C) It is tetrahedral because there are two bonded pairs and two lone pairs around oxygen.
D) It is bent because there are two bonded pairs and two lone pairs around oxygen.
Answers
Answer: D
Explanation:
Water is comprised of 2 Hydrogen atoms and 1 Oxygen atom. This gives us a total of 8 valence electrons to form bonds with.
Because Hydrogen is in first row of elements it can only hold a total of two electrons. This means each Hydrogen holds 2 electrons.
But that means there are four more electrons. VSEPR theory is basically how electron repulsion causes atoms to arrange in different ways.
There will be four electrons left on the oxygen because they can not go anywhere else. This will cause the hydrogen molecules to move away from the lone electrons and cause a bent geometry rather than a straight-line geometry.
If star a appears to move back and forth by a greater amount than star b, which star is closer to you?
Answers
If Star A appears to move back and forth by a greater amount than Star B, which star do you think is actually closer to you? Star A. If the parallax angle for a star is 1 arcsecond, what is the distance from the Sun to that star
please hurry asap I WILL MARK BRAINLEST
The Statue of Liberty is made out of copper and was once shiny and copper-colored. Over the years, the copper has gone through a process called oxidation, where it has changed into a new material.
What has occurred?
an endothermic reaction
an energy formation
a chemical reaction
a precipitate formation
Answers
Answer:
an endothermic reaction
Explanation:
An endothermic reaction has occurred.
Answer:
endothermic
Explanation:
took the quiz
what is mean by exhaustible natural resources of list some
Answers
Answer:
enr are those resources which are present in limited quality and can be use completely used up by human activities are called enr .example coal petrol
How many
sulfur dioxide
molecules are in 2.8 moles of
sulfur dioxide?
[?]×10l21
Answers
The find the number of sulfur dioxide molecules in 2.8 moles of sulfur dioxide, we need to use Avogadro's number. Avogadro's number is defined as the number of atoms, molecules, or particles in one mole of a substance, which is approximately 6.022 × 10²³.
The First, we need to calculate the total number of moles of sulfur dioxide in 2.8 moles. Since sulfur dioxide has a molecular formula of SO₂, each molecule contains one sulfur atom and two oxygen atoms. Therefore, the molar mass of sulfur dioxide is 32.06 g/mol for sulfur plus (2 x 16.00 g/mol) for oxygen, which is equal to 64.06 g/mol. So, 2.8 moles of sulfur dioxide will have a mass of: 2.8 mol x 64.06 g/mol = 179.37 g Now, we can use Avogadro's number to find the total number of molecules in 2.8 moles of sulfur dioxide Number of molecules = Number of moles x Avogadro's number of molecules = 2.8 mol x 6.022 × 10²³ molecules/mol Number of molecules = 1.686 × 10²⁴ molecules Therefore, there are approximately 1.686 × 10²⁴ sulfur dioxide molecules in 2.8 moles of sulfur dioxide.
learn more about dioxide here.
https://brainly.com/question/15153008
#SPJ11
A 475 cm3 sample of gas at standard temperature (273 K) and pressure
(1 atm) is allowed to expand until it occupies a volume of 600. cm3. What
temperature would be needed to return the gas to standard pressure (1 atm)?
(n = constant
Answers
Answer:
T₂ = 344.8 K
Explanation:
Given data:
Initial volume of gas = 475 cm³
Initial temperature = 273 K
Final volume = 600 cm³
Final temperature = ?
Solution:
The given problem will be solve through the Charles Law.
According to this law, The volume of given amount of a gas is directly proportional to its temperature at constant number of moles and pressure.
Mathematical expression:
V₁/T₁ = V₂/T₂
V₁ = Initial volume
T₁ = Initial temperature
V₂ = Final volume
T₂ = Final temperature
Now we will put the values in formula.
V₁/T₁ = V₂/T₂
T₂ = T₁V₂/V₁
T₂ = 273 K × 600 cm³ / 475 cm³
T₂ = 163800K . cm³ / 475 cm³
T₂ = 344.8 K
A 7.0 liter balloon at room temperature contains helium gas. if the temperature is halved, what happen to the volume?
Answers
The volume = 6.71 L
Further explanationCharles's Law
When the gas pressure is kept constant, the gas volume is proportional to the temperature
\(\tt \dfrac{V_1}{T_1}=\dfrac{V_2}{T_2}\)
V₁=7 L
T₁=25 °C(room temperature) + 273 = 298 K
T₂=12.5(halved) + 273 = 285.5 K
\(\tt \dfrac{V_1}{T_1}=\dfrac{V_2}{T_2}\\\\\dfrac{7}{298}=\dfrac{V_2}{285.5}\\\\V_2=6.71~L\)
can anybody plz hep me on this



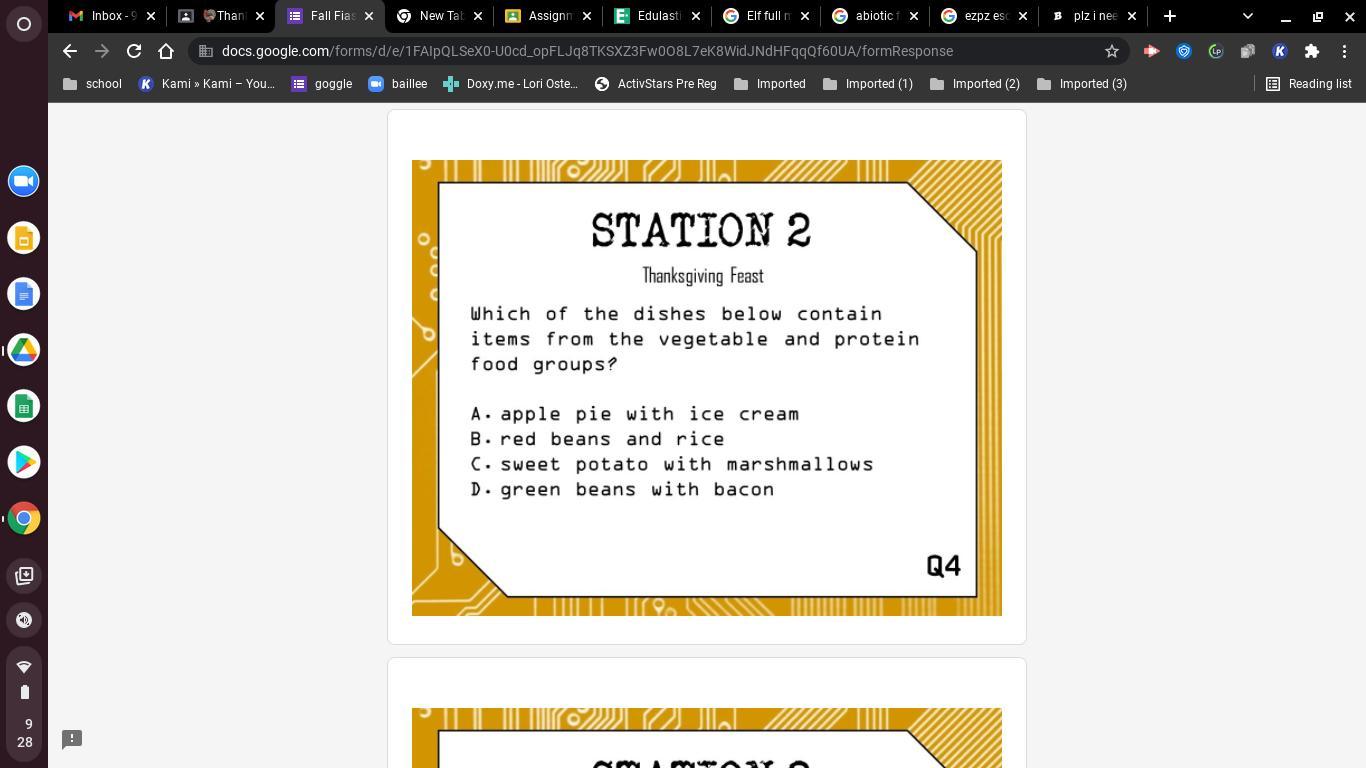

Answers
C is for the digestion questions
B is for the food question
A is the muscle question for stomach
I’m pretty sure
A friend suggests the following question for a science experiment. Discuss ways that the question could be reworded to make it a more appropriate scientific question. How much pollution is in urban streams?
Answers
Answer:
I don't think so
Explanation:
many pollution
how to determine if a gas is monoatomic or diatomic by the ratio of specific heat when pressure is constant and volume
Answers
By comparing the measured value of γ to the known values for monoatomic and diatomic gases, we can determine the nature of the gas and whether it is monoatomic or diatomic.
To determine if a gas is monoatomic or diatomic based on the ratio of specific heat at constant pressure (Cp) to specific heat at constant volume (Cv), we can use the following relationship:
γ = Cp / Cv
Where:
γ is the ratio of specific heat, also known as the adiabatic index or heat capacity ratio.
For monoatomic gases, such as noble gases like helium (He) and argon (Ar), the ratio of specific heats is γ = 5/3 ≈ 1.67.
For diatomic gases, such as nitrogen (N₂), oxygen (O₂), and hydrogen (H₂), the ratio of specific heats is γ = 7/5 = 1.4.
By measuring the ratio γ experimentally, we can determine whether the gas is monoatomic or diatomic.
If the measured value of γ is approximately 1.67, it suggests that the gas is monoatomic.
If the measured value of γ is approximately 1.4, it suggests that the gas is diatomic.
This difference in the ratio of specific heat arises from the degrees of freedom in the molecular structure of the gas. Monoatomic gases have three degrees of freedom, while diatomic gases have five degrees of freedom.
Learn more about diatomic at https://brainly.com/question/11446176
#SPJ11
45. The following data was collected for 3 compounds:
Mass of Nitrogen that combines with 1 g of Oxygen
Compound A 1.750 g
Compound B 0.8750 g
Compound C 0.4375 g
Show whether these are the same or different compounds. What chemical law is being observed here?
Answers
Answer:
The three compounds are different compounds
Explanation:
The mass of Nitrogen that combines with 1 gram of Oxygen in Compound A = 1.750 g
The mass of Nitrogen that combines with 1 gram of Oxygen in Compound B = 0.8750 g
The mass of Nitrogen that combines with 1 gram of Oxygen in Compound C = 0.4375 g
According to the law of multiple proportions, when atoms of two different elements react to form compounds, the masses of one of the elements that combines with a fixed mass of the other element are in small whole number ratios.
The ratio of the masses are;
Mass of Nitrogen in Compound B/(Mass of Nitrogen in Compound C = 0.8750/0.4375 = 2
Mass of Nitrogen in Compound A/(Mass of Nitrogen in Compound C = 1.750/0.4375= 4
Mass of Nitrogen in Compound A/(Mass of Nitrogen in Compound B = 1.750/0.8750= 2
Given that the masses of Nitrogen in the three compounds are in small whole number ratios, the three compounds, Compound A, Compound B, and Compound C are different compounds.
What is the mass of water released by the heating? Show your work or explain your reasoning.
Answers
Answer:
Dividing the mass of the water lost by the original mass of hydrate used is equal to the fraction of water in the compound. Multiplying this fraction by 100 gives the percent water in the hydrate.
Explanation:
The amount of water throughout the compound has been determined by dividing the mass of water wasted mostly by original quantity of hydrate used. The above fraction can be multiplied by 100 to get the hydrate's water content in percentages.
What is mass ?The proportion of matter that makes up an object is quantified by its mass. The kilogram, or kg, would be the fundamental SI unit of mass.
What is hydrate?Any substance that contains water through the form of H2O molecules is referred to as a hydrate. This water content by weight can vary, but it is typically fixed. The most well-known hydrates seem to be crystalline solids which decompose once the attached water is removed.
Therefore , The amount of water throughout the compound has been determined by dividing the mass of water wasted mostly by original quantity of hydrate used. The above fraction can be multiplied by 100 to get the hydrate's water content in percentages.
To know more about mass and hydrate.
https://brainly.com/question/11202174
#SPJ3