Enough of a monoprotic acid is dissolved in water to produce a 0.0158 m solution. the ph of the resulting solution is 2.64. calculate the a for the acid.
Answers
PH = 2.64
PH = - log [H+]
[H+] = 0.00229 M
HA = 0.0199 M
Dissociation equation
HA --> H+ + A-
Ka = [H+][A-]/[HA]
[H+] = [A-]
Ka = (0.00229^2)/0.0158
=3.31 x 10^-4M
What is monoprotic acids?
Any acid with only one hydrogen atom in its formula is called a monoprotic acid, while some acids with multiple hydrogen atoms may also fall under this category. In other words, while all monoprotic acids contain only one hydrogen, not all single-hydrogen acids are monoprotic.
Therefore,
Enough of a monoprotic acid is dissolved in water to produce a 0.0158 m solution. the ph of the resulting solution is 2.64. calculate the a for the acid.
pH = 2.64
pH = - log [H+]
[H+] = 0.00229 M
HA = 0.0199 M
Dissociation equation
HA --> H+ + A-
Ka = [H+][A-]/[HA]
[H+] = [A-]
Ka = (0.00229^2)/0.0158
=3.31 x 10^-4M
To learn more about monoprotic acid from the given link:
https://brainly.com/question/3006391
#SPJ4
Related Questions
hcn and kcn have similar chemical formulas. however, 0.1 m hcn has a ph of 5.2, while 0.1 m kcn has a ph of 11.2. why do these two compounds behave so differently when they dissolve in water? hcn has a great solubility. hcn is an acid, and kcn is a salt. kcn is much stronger base than hcn. hcn is a nonelectrolyte.
Answers
HCN is highly soluble in water,
With rising temperatures and in extremely saline environments, its solubility declines. HCN is a colorless gas and liquid with an odor reminiscent to bitter almonds, however not everyone can smell it. HCN is created in solution when the cyanide ion interacts with water.
What is HCN?
Prussic acid, also known as hydrogen cyanide, is a chemical substance having the formula HCN and the structural formula HCN. It is a colorless, very deadly, and combustible liquid that boils at 25.6 °C (78.1 °F), only barely over room temperature. Industrial-scale HCN production makes it a highly prized precursor to a wide range of chemical compounds, from medications to polymers. Production of potassium cyanide and adiponitrile, which are used in mining and polymers, respectively, has large-scale uses. Due to its liquid nature, it is more hazardous than cyanide compounds that are solid.
To know more about Prussic acid, visit:
https://brainly.com/question/13326666
#SPJ4
considering the frequency of non-polar bonds in each type of molecule, which type of molecule should have the most potential energy (assuming you have the same mass of each)?
Answers
Considering the frequency of non-polar bonds in each type of molecule, the molecule with the most potential energy should be the one with the highest number of non-polar bonds.
Non-polar bonds are stronger than polar bonds and so molecules with more of these bonds can store more energy due to their increased stability. For example, molecules with higher amounts of carbon-carbon and carbon-hydrogen bonds tend to have higher potential energies than molecules with more polar bonds such as oxygen-hydrogen bonds. Non-polar bonds are bonds between two atoms that have the same electronegativity, such as two atoms of carbon or two atoms of hydrogen. These bonds do not have a charge distribution and no molecules are attracted to them, which makes them non-polar. Polar bonds, on the other hand, are bonds between two atoms with different electronegativities, such as oxygen and hydrogen or nitrogen and hydrogen. These bonds have a partial charge distribution, which means that molecules are attracted to them, and this makes them polar.
To know more about electronegativity please refer:
https://brainly.com/question/17762711
#SPJ4
how many columns of elements does the periodic table contain
Answers
The periodic table contains 18 columns of elements, also known as groups or families.
The periodic table is a tabular arrangement of chemical elements organized based on their atomic number, electron configuration, and chemical properties. It consists of rows called periods and columns called groups or families.
1. Groups or Families: The columns in the periodic table are known as groups or families. Each group contains elements that share similar chemical properties and exhibit similar patterns in their electron configurations. The elements within a group have the same number of valence electrons in their outermost energy level.
2. Number of Groups: The modern periodic table consists of 18 groups labeled from 1 to 18. These groups are further divided into several subgroups based on the filling of different types of orbitals.
3. Representative Elements: The first two groups on the left side of the periodic table are known as the s-block elements, and the next six groups are referred to as the p-block elements. Together, these groups make up the representative elements, which include elements from hydrogen (H) to helium (He) and from boron (B) to neon (Ne) in the first and second periods.
4. Transition Metals: Following the representative elements are the transition metals, occupying the d-block in the periodic table. They consist of ten groups labeled from 3 to 12.
5. Inner Transition Metals: At the bottom of the periodic table are the inner transition metals, which are further divided into two rows known as the lanthanides (rare earth elements) and the actinides. These elements are labeled as groups 3 to 12 and 13 to 18.
In summary, the periodic table contains 18 columns of elements, known as groups or families, each with a unique set of chemical properties and electron configurations. These groups help organize and categorize the elements based on their shared characteristics and trends in properties.
To know more about periodic table refer here:
https://brainly.com/question/28747247#
#SPJ11
Which electron in an atom (Z=17) is the most shielded from
nuclear charge?
A. an electron in the 2s
B. an electron in the 3p
C. an electron in the 3d
D. an electron in the 1s
Answers
Answer:
option no. C is correct
an electron in the 3d
Kate and Janie are about to pour water into a beaker when they notice
the beaker has a crack at the bottom. What should the students do
with the beaker?
Answers
Answer:
Put the beaker in the broken glassware disposal and replace the beaker
Explanation:
Any beaker which has a crack could break/shatter if you apply heat. Also, cracked glassware is a lab safety hazard.
what is the concentration of a household ammonia cleaning solution if 49.90 ml of 0.5900 m HCl is required to neutralize 25.00 ml of the solution?
Answers
Answer:
The balanced chemical equation for the neutralization reaction between ammonia (NH3) and hydrochloric acid (HCl) is:
NH3 + HCl → NH4Cl
From this equation, we can see that 1 mole of NH3 reacts with 1 mole of HCl to produce 1 mole of NH4Cl.
To find the concentration of the household ammonia cleaning solution, we can use the following formula:
M1V1 = M2V2
where M1 is the concentration of the HCl solution, V1 is the volume of the HCl solution used, M2 is the concentration of the ammonia solution, and V2 is the volume of the ammonia solution used.
Plugging in the given values, we get:
M1 = 0.5900 M
V1 = 49.90 mL = 0.04990 L
V2 = 25.00 mL = 0.02500 L
We can solve for M2:
M2 = (M1V1) / V2
M2 = (0.5900 M)(0.04990 L) / 0.02500 L
M2 = 1.17 M
Therefore, the concentration of the household ammonia cleaning solution is 1.17 M.
Explanation:
A thick, oily liquid found deep in the earth that is refined and used as a source for gasoline is called _____________?
Petroleum
Reserve
Lumber
Coal
Answers
It would be A, Petroleum.
Hope this helped!
-William
Which material is the limiting ingredient if you have 2 cans of tomatoes, 2 cups green beans, 2 cups pasta, 8 cans of beans, and 12 cups broth
2 cans diced tomatoes
1 cup green beans
1/2 cup pasta
1 can beans
4 cups broth
Answers
Based on the recipe for preparing the food, the limiting ingredient is the 2 cans of tomatoes.
What is the recipe for the food?The recipe for the food is given below:
2 cans diced tomatoes1 cup green beans1/2 cup pasta1 can beans4 cups brothHence, 2 cans of diced tomatoes require 1 cup of green beans, 1/2 cup of pasta, 1 can of beans, and 4 of cups broth.
Hence, after, the 2 cans of diced tomatoes are used up, no more food can be prepared.
A limiting reagent or limiting ingredient is used up when cooking, hence, the 2 cans of tomatoes are the limiting ingredient.
Learn more about limiting reagent at; https://brainly.com/question/23661051
#SPJ1
difference between practical work inside and outside laboratory
Answers
Practical work refers to the art of conducting experiments in order to answer certain research questions.
What is practical work?In science, practical work refers to the art of conducting experiments in order to answer certain research questions. This could occur in a laboratory under controlled conditions or in the field.
In the physical sciences, most of the practical work is conducted in the laboratory under controlled conditions. However, some experiments in the biological sciences and most experiments in the social sciences are conducted outside the laboratory.
Learn more about experiments:https://brainly.com/question/11256472
#SPJ1
Consider an FCC unit cell with a lattice constant, a, of 3.2A˚. Determine the surface atomic concentration of the (111) plane in this crystal.
Answers
An FCC unit cell with a lattice constant, a, of 3.2A˚. The surface atomic concentration of the (111) plane in this crystal is 0.2202 atoms/Ų.
To determine the surface atomic concentration of the (111) plane in an FCC crystal, we need to consider the arrangement of atoms on that plane.
In an FCC crystal, the (111) plane consists of a triangular array of atoms. Each triangular arrangement contains one atom at the center and three atoms at the corners.
To calculate the surface atomic concentration, we need to determine the number of atoms on the (111) plane per unit area. This can be done by considering the area of the unit cell and the arrangement of atoms.
The area of the unit cell in the (111) plane can be calculated using the lattice constant, a. Since the (111) plane is a triangular plane, the area of the unit cell in that plane is given by:
Area = (√(3) * a²) / 2
To determine the number of atoms on the (111) plane. Each triangular arrangement on the (111) plane contains one atom at the center and three atoms at the corners.
Therefore, the total number of atoms on the (111) plane per unit cell is:
Number of Atoms = 1 (central atom) + 3 (corner atoms) = 4
Calculate the surface atomic concentration by dividing the number of atoms on the (111) plane by the area of the unit cell in that plane:
Surface Atomic Concentration = Number of Atoms / Area
Substituting the values, we have:
Surface Atomic Concentration = 4 / [(√(3) * a²) / 2]
Given that the lattice constant, a, is 3.2 Å, we can calculate the surface atomic concentration as follows:
Surface Atomic Concentration = 4 / [(√(3) * 3.2²) / 2]
Surface Atomic Concentration = 0.2202 atoms/Ų
Therefore, the surface atomic concentration of the (111) plane in the given FCC crystal with a lattice constant of 3.2 Šis approximately 0.2202 atoms/Ų.
To know more about atomic concentration here
https://brainly.com/question/33356126
#SPJ4
4. 100 mL of an NaOH solution is neutralized by 50 mL of a 0.5M HCl solution. What is the molarity of the NaOH solution?
Please help with real answer
Answers
Answer:
6.098M
Explanation:
akording to m1v1/m2v2=n1/n2
How could the winds flowing in a certain direction affect air masses and where they may collide?
Answers
Answer:
becasue they are coming from both sides
Explanation:
The air masses gets lift up by the winds flowing in certain direction. Air masses collide when the air becomes humid and wind speed is greater.
What are winds ?Winds are passage of air from high pressure area to low pressure area. Air masses may travel from one place to another as a result of variations in air pressure. Air masses often move from high-pressure regions to low-pressure regions.
Winds therefore sweep away from high-pressure zones and toward low-pressure ones. The winds can moves the air masses in the atmosphere and the air masses collide in the clod and humid air.
Winds transfer meteorological conditions from the source location to the destination zone as they move air masses. The air mass may collide with another air mass that has a different temperature and humidity as it enters a new area. This may bring about a powerful storm.
Find more on air mass:
https://brainly.com/question/12089291
#SPJ2
How formaldehyde is formed?
Answers
In addition to other natural and human-made processes, the burning of organic materials is the main source of formaldehyde formation.
The simplest of the aldehydes, formaldehyde (HCHO), commonly known as methanal, is an organic molecule utilized in huge quantities during numerous chemical industrial processes. It is often supplied as formalin, an aqueous solution with a 37 percent concentration, and is primarily manufactured via the vapor-phase oxidation of methanol.
Industrial production of formaldehyde involves the catalytic oxidation of methanol. The most often used catalysts are iron(III) oxide, iron molybdenum oxides, and silver metal [e.g. Vanadium oxides or iron(III) molybdate with a molybdenum-enriched surface. Methanol and oxygen react at around in the commonly used formox process.
For more information on formaldehyde kindly visit to
https://brainly.com/question/13763519
#SPJ4
If astronauts landed on a planet 2/3 the size of earth what would it be
Answers
Answer: The ratio of radii of earth to another planet is 2/3 and the ratio of their mean densities is 4/5. If an astronaut can jump to a maximum height of 1.5 m on the earth, with the same effort, the maximum height he can jump on the planet is
Explanation:
What role do stomach enzymes play in digestion? A They transfer food out of the esophagus. B They break down food into smaller parts. C They move food down into the intestines. D They physically mix food with stomach acid.
Answers
Answer:
b
Explanation:
Answer:
b
Explanation:
b
Which object has the most kinetic energy?
A. A charged battery sitting in a kitchen drawer.
B. A pear falling from the top branch of a tree.
C. A swimmer sitting on a diving board at the pool.
D. A roller coaster rolling down a steep track.
Apex~
Answers
Answer:
D
Explanation:
I think the answer is part d.
How many neutrons are in the nucleus of a potassium atom with a mass number of 39?.
Answers
Answer:
20 neutrons
Explanation:
neutrons = mass number - atomic number
mass number = 39, atomic number = 19
neutrons = 39 - 19 = 20
is the maximum population that a given area can support.
Carrying capacity
Population growth
Limiting factor
Immigration rate
Answers
The carrying capacity is the maximum number of individuals of a species that an environment can support. Population size decreases above carrying capacity due to a range of factors depending on the species concerned, but can include insufficient space, food supply, or sunlight.
Scientists often need to represent very small numbers and very large number, which have many digits. These number can be so long that they are difficult to read. So, scientists developed a simpler method to represent these numbers, called
Answers
Answer:
Scientific notation
Explanation:
For example 10000000000 =10 to the power of ten
help help help help help

Answers
Answer:
It's C.
Explanation:
According to the site, "http://needtoknow.nas.edu/energy/energy-sources/renewable-sources/wind/" ... "Wind energy is an indirect form of solar energy created by a combination of factors, including the uneven heating of Earth’s atmosphere by solar radiation, variations in topography, and the rotation of Earth. People have been putting wind energy to use throughout history to propel sail boats, mill flour from grain, and pump water."
Diffusion and Organelle Retake Activity
(please finish in 5-9 minutes)
Question:
Fill in the blank
Water loves _____ and ____.
This means water will
move ______ the direction of the salt or sugar.
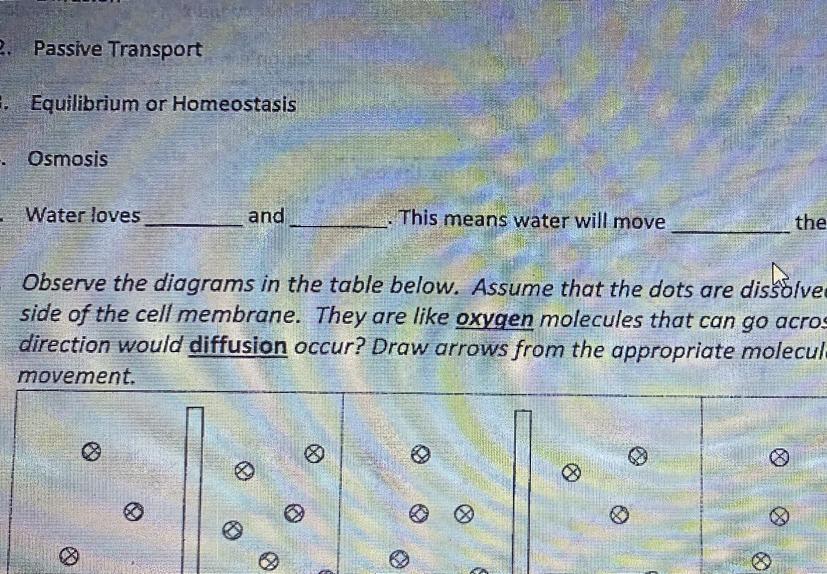
Answers
Answer:
wind and soil? north?
Explanation:
what happens when the drop of food coloring enters the water
Answers
When a drop of food coloring enters the water, several processes occur:
1. Diffusion: This is the main process. Molecules of food coloring move from an area of higher concentration (the drop) to an area of lower concentration (the water). They spread out to evenly distribute themselves throughout the water.
2. Advection: If the water is moving (for example, if you stir it), this can carry the food coloring along with it.
3. Convection: If there are temperature differences within the water, these can create currents that move the food coloring around.
Eventually, assuming no other forces are acting on the water (like stirring), the food coloring will evenly distribute itself throughout the water due to the process of diffusion. This is a passive process that doesn't require any energy, as it's powered by the random motion of the molecules.
By shining a light on an electron, you can guarantee that the electron will not be where you are looking for it.
A. True
B. False
The _______ quantum number tells you the shape of the electron cloud.
A. Principal
B. Azimuthal
C. Magnetic
D. Spin
Thank you
Answers
Answer:
A. True
Explanation:
But, it depends on the brightness of the light hitting on the metal surface.
Answer:
B. Azimuthal
Explanation:
The Azimuthal quantum number tells you the shape of the electron cloud. It is also known as the orbital angular momentum quantum number.
what is the third quantum number of a 3 s 2 electron in phosphorus, 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3 ?
Answers
The third quantum number (m_l) of a 3s² electron in phosphorus is 0.
The third quantum number, denoted as m_l, represents the magnetic quantum number and describes the orientation of an orbital within a subshell. It can have integer values ranging from -l to +l, where l is the azimuthal quantum number.
In the electron configuration of phosphorus, we see that the 3s subshell is being filled. The azimuthal quantum number (l) for the 3s subshell is 0. Since the electron is in the 3s² subshell, there are two electrons present in the 3s orbital.
For the two electrons in the 3s orbital, they will have opposite spins due to the Pauli exclusion principle. However, the magnetic quantum number (m_l) for both electrons in the 3s orbital will be the same, which is 0.
Therefore, the third quantum number (m_l) of a 3s² electron in phosphorus is 0. This means that both electrons in the 3s orbital have the same orientation within the subshell.
To learn more about quantum number, here
https://brainly.com/question/31955577
#SPJ4
What is ionic bond and explain it
Answers
Answer:
An ionic bond is a chemical bonding involving the attraction between oppositely charged ions
Explanation:
On the periodic table, elements from group 1 and 7 are attracted to each other and when they bond, it's called ionic bonding. This is because of their valence electrons and ions.
Answer:
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. It is one of the main types of bonding along with covalent bonding.
NEED HELP‼️‼️‼️
What would happen if strong force and electromagnetic force was reversed?
Answers
Answer:
Since electromagnetic forces are responsible for regular chemical bonds, every chemical compound would dissolve. All life and all ordinary objects would cease to exist. I think your question would call on electrons and protons to cease to exist, since these particles are associated with the electromagnetic force.
Explanation:
what type of fat is created when hydrogen is added to liquid oil?
Answers
When hydrogen is added to liquid oil, it creates a type of fat known as hydrogenated fat. This process is called hydrogenation, and it is often used in food manufacturing to make products like margarine, shortening, and some types of snack foods.
Hydrogenated fats have a longer shelf life and are more stable at high temperatures than liquid oils, making them useful for baking and frying. However, hydrogenated fats also contain high levels of trans fats, which are known to increase the risk of heart disease.
In recent years, many food manufacturers have switched to using other types of fats, such as palm oil or canola oil, to avoid the negative health effects associated with trans fats. In general, it is recommended to limit the consumption of foods high in hydrogenated fats and trans fats to maintain good health.
To know more about hydrogenated visit:
https://brainly.com/question/14618125
#SPJ11
The radioisotope phosphorus-32 is used in tracers for measuring phosphorus uptake by plants. The half-life of phosphorus-32 is 14.3 days. How much time is required for the activity of a sample of phosphorus-32 to fall to 7.34 percent of its original value
Answers
Answer:
54 days
Explanation:
We have to use the formula;
0.693/t1/2 =2.303/t log Ao/A
Where;
t1/2= half-life of phosphorus-32= 14.3 days
t= time taken for the activity to fall to 7.34% of its original value
Ao=initial activity of phosphorus-32
A= activity of phosphorus-32 after a time t
Note that;
A=0.0734Ao (the activity of the sample decreased to 7.34% of the activity of the original sample)
Substituting values;
0.693/14.3 = 2.303/t log Ao/0.0734Ao
0.693/14.3 = 2.303/t log 1/0.0734
0.693/14.3 = 2.6/t
0.048=2.6/t
t= 2.6/0.048
t= 54 days
Write the following in standard notation: 2.8 x 10-5s.
Answers
Answer:
2.8 X 10-5s in standard notation is 28-5s
Explanation:
Multiply 2.8 by 10
.What is the charge of the ion most commonly formed by f?
- (−)
- 2−
- (+)
- 2+
Answers
The ion most commonly formed by fluorine is the fluoride ion (F-). This ion has a charge of -1, meaning it has gained an electron to form an anion.
Fluorine has a high electronegativity, meaning it is highly likely to attract an electron to complete its outer shell and become more stable. As a result, the fluoride ion is formed when fluorine gains an electron.
to know more about solubility intake pls visit:
https://brainly.com/question/22819229
#SPJ11