Answers
Answer:
The atomic number stands for the number of protons in an atom of the element.
The number of protons determines the identity of the element,
So the atomic number identifies the element. GOOD LESSONS ♡
Related Questions
For the reaction
C₂H₂(g)+30₂(g) → 2CO₂(g) +2H₂O(g)
if 5.0 mol of CO₂ are produced, how many moles of O₂ were reacted?
(A) 12.5 mol
(B) 3.3 mol
C)7.5 mol
D)none of these
(E) 6.2 mol
I think it’s C
Answers
Answer: C
Explanation: 3 mol O2 ⇒ 2 mol CO2
O2 reacts to produce 5 mol of CO2 = (3 * 5)/2 = 7.5 mol O2
If a solution has a [H+] concentration of 4.5 x 10-7 M, is this an acidic or basic solution?
Solve and Explain.
Answers
Considering the definition of pH, the pH is 6.35 and the solution is acidic.
Definition of pHpH is the Hydrogen Potential and it is a measure of acidity or alkalinity. pH indicates the amount of hydrogen ions present in a solution or substance.
Mathematically, pH is calculated as the negative base 10 logarithm of the activity of hydrogen ions:
pH= - log [H⁺]
The numerical scale that measures the pH of substances includes the numbers from 0 to 14. The pH value 7 corresponds to neutral substances. Acidic substances are those with a pH lower than 7, while basic substances have a pH higher than 7.
Acidic or basic solution in this caseIn this case, being [H⁺]=4.5×10⁻⁷ M, you can replace this value in the definition of pH:
pH= -log (4.5×10⁻⁷ M)
Solving:
pH= 6.35
Finally, the pH is lower than 7, the solution is acidic.
Learn more about pH:
brainly.com/question/3992824
#SPJ1
Liquid hexane CH3CH24CH3 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O. Suppose 4.3 g of hexane is mixed with 25.8 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Round your answer to 2 significant digits.
Answers
Answer:
13g of CO₂ is the maximum amount that could be produced
Explanation:
The reaction of hexane with oxygen is:
C₆H₁₄ + 19/2O₂ → 6CO₂ + 7H₂O
Where 19/2 moles of oxygen react per mole of C₆H₁₄
To solve this question we need to find theoretical yield finding limiting reactant :
Moles C₆H₁₄:
4.3g C₆H₁₄ * (1mol / 86.18g) = 0.0499 moles
Moles O₂:
25.8g * (1mol / 32g) = 0.806 moles
For a complete reaction of 0.0499 moles of C₆H₁₄ are needed:
0.0499 moles of C₆H₁₄ * (19/2 mol O₂ / 1mol C₆H₁₄) = 0.474 moles of O₂.
As there are 0.806 moles, O₂ is in excess and C₆H₁₄ is limiting reactant
In theoretical yield, the moles of hexane added = 6Moles of CO₂ produced. Moles of CO₂ are:
0.0499 moles C₆H₁₄ * (6mol CO₂ / 1mol C₆H₁₄) = 0.299 moles CO₂
In grams:
0.299 moles CO₂ * (44.01g / mol) = 13g CO₂
13g of CO₂ is the maximum amount that could be produced
How many grams are there in 4.4 moles of calcium, Ca?
Answers
Answer:
180 g Ca
General Formulas and Concepts:
Chemistry - Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisExplanation:
Step 1: Define
4.4 mol Ca
Step 2: Identify Conversions
Molar Mass of Ca - 40.08 g/mol
Step 3: Convert
\(4.4 \ mol \ Ca(\frac{40.08 \ g \ Ca}{1 \ mol \ Ca} )\) = 176.352 g Ca
Step 4: Check
We are given 2 sig figs. Follow sig fig rules and round.
176.352 g Ca ≈ 180 g Ca
Red paint mixed with blue paint becomes purple. What is the most likely cause of this color change?
A.
a change in the state of matter
B.
the formation of a new substance
C.
a physical change
D.
a chemical reaction
plz help
Answers
Answer:
I might be late but when red paint and blue paint mix to become purple. its a physical change, so its C
Explanation:
5. From the two examples provided, would you expect the formula Sg to represent a compound to an element?
Answers
From the two examples provided, the chemical formula Sg represents an element.
What is an element?
It is defined as a substance which cannot be broken down further into any other substance. Each element is made up of its own type of atom. Due to this reason all elements are different from one another.
Elements can be classified as metals and non-metals. Metals are shiny and conduct electricity and are all solids at room temperature except mercury. Non-metals do not conduct electricity and are mostly gases at room temperature except carbon and sulfur.
Learn more about elements,here:
https://brainly.com/question/13516179
#SPJ1
part c.1. the water level in the co2(g)-collection cylinder is higher than the water level outside the cylinder. see margin drawing. (page 188).
Answers
Because a higher water level translates into a lower atmospheric pressure, the wet CO2 gas pressure is lower than the atmospheric pressure.
Wet CO2 gas pressure is therefore lower than air pressure outside the collection cylinder if the water level inside is higher than the water level outside.
Why does gas accumulate over water?By displacing water, a gas created during a chemical reaction can be collected. As a result of being gathered over water, the gas is not pure and is instead combined with water vapor. By deducting the contribution of the water vapor, the amount of the desired gas can be calculated using Dalton's law.
To know more about pressure visit:-
https://brainly.com/question/29341536
#SPJ4
A 5 kg ball is traveling at the same speed as a 10 kg ball. Compared to with 5 kg ball, the 10 kg ball has (2 points)
Answers
Answer: twice the momentum
Explanation:
Which choice is not an example of a molecule?
A) 03
B) Mn
C) KOH
D)H₂S
Answers
I did it wrong but I just can’t figure it out.


Answers
(a) The number of moles of Ag⁺ that reacted is 0.05 mmoles and moles of Cl- that reacted is 0.05 mmoles.
(b) The number of moles of AgCl(s) formed is 0.05 moles
(c) The molarity of Ag⁺ after the reaction = 0.0 M
(d) The molarity of NO₃⁻ after the reaction is 0.25 M
What is the number of moles of Ag and Cl- that reacted?The number of moles of Ag and Cl- that reacted is calculated as follows from the equation of the reaction:
AgNO₃ (aq) + NaCl (aq) ---> AgCl (s) + NaNO₃ (aq)
(a) The number of moles of Ag and Cl- that reacted:
Moles of Ag⁺ = 0.5 * 0.1
Moles of Ag⁺ = 0.05 mmoles
Moles of Ag⁺ that reacted = 0.05 mmoles
Moles of Cl⁻ = 0.5 * 0.1
Moles of Cl⁻ = 0.05 mmoles
Moles of Cl⁻ that reacted = 0.05 mmoles
(b) The number of moles of AgCl(s) formed.
Moles of AgCl that formed = 0.05 moles
(c) Since there are no more Ag⁺ ions in the mixture, the molarity of Ag⁺ after the reaction = 0 moles
(d) The molarity of NO₃⁻ after the reaction.
Moles of NO₃⁻ = 0.5 * 0.1
Moles of NO₃⁻ = 0.05 moles
Volume of mixture = 0.2 mL
The molarity of NO₃⁻ after the reaction = 0.05/0.2
The molarity of NO₃⁻ after the reaction = 0.25 M
Learn more about molarity at: https://brainly.com/question/30404105
#SPJ1
Question 3 (11 points)
A gas has a volume of 690.0mL at -15.1°C and 392.0 mmHg. What would the volume of the gas be at
233.0°C and 0.700 atm of pressure? Answer with no decimal places.
Answers
Answer:
V2 = 0.998L
v2 = 0.000998mL
Explanation:
P1 = 392mmHg
V1 = 690mL
T1 = -15.1°C = 257.9k
P2 = 0.700atm = 532mmHg
V2 = ?
T2 = 233°C = 506K
using general gas equation
P1V1/T1 = P2V2/T2
(392 x 690.0 x 10^-³)/257.9 =(532 x V2)/506
1.05=(532 x V2)/506
1.05 x 506 =(532 x V2)
531.3 =(532 x V2)
V2 = 531.3/532
V2 = 0.998L
v2 = 0.000998mL
Calcium nitrate- make an evidence based argument for why acid rain would cause the calcium carbonate in the marble to slowly break down and wash away over time when calcium nitrate is produced
Answers
Following are the effects of acid rain.
What is Acid Rain?
Acid rain is caused by the accumulation of nitric and sulfuric acids in the atmosphere. These compounds are strong acids and are very soluble in water, dissolving in droplets in clouds.
Limestone effect:
Calcium carbonate, [Ca][CO3], is a very common mineral. A limestone is a well-known form of calcium carbonate. The acid contained in acid rain reacts with carbonate ions and promotes the dissolution of calcium carbonate.
This will create a bicarbonate solution. The presence of limestone and other calcium carbonates in lakes and streams helps maintain a constant pH as the mineral reacts with excess acidity. However, acid rain can eventually exceed the buffering capacity of surface waters.
How does acid rain affect buildings made of marble and limestone?
It is mainly affected in 2 ways: dissolution and alteration. Calcite dissolves when sulfuric, sulfuric and nitric acids in polluted air react with calcite in marble and limestone. Exposed areas of buildings and statues show rough surfaces, worn materials, and loss of sculptural detail. Stoneface material can be lost anywhere or only in more reactive areas.
Hence, this is how acid rain affects.
To learn more about Acid Rain, click on the given link: https://brainly.com/question/718250
#SPJ1
What is the term used by particular kind of matter Called??
Answers
Answer:
\(\boxed{\mathrm{substance}}\)
Explanation:
The term used by particular kind of matter is called substance.
A substance is a particular kind of matter because it has physical properties.
If a new McDonalds opens up in town, then the supply curve for cheeseburgers will shift to
to the left
to the right
upward
downward
Answers
HF+H2o reacts to form ??
Answers
Answer:
Explanation:
HF + H2O reacts to form H3O+ (hydronium ion) and F- (fluoride ion).
Anything that has mass and takes up space is what
0)
A
tass
B)
volume
)
density
D)
matter
Answers
Answer:
D - Matter
Explanation:
Matter is defined as anything that has mass and takes up space. Matter is basically liquid soilds and gasses!
(I hope this helps :>)
Predict the products for the following reactions (there are TWO products)
Li(s) + KNO3 (aq) →
Options:
LI
LiNO3
KNO3
K
Li₂NO3
Li(NO3)2
Answers
An equation which obey the law of conservation of mass is defined as the balanced chemical equation. According to law of conservation of mass, mass can neither be created nor be destroyed. The products are LiNO₃ and K. The correct options are B and D.
The substance which appears on the left hand side of the equation is defined as the reactants whereas the substance which are present on the right hand side are called products.
The reaction between 'Li' and KNO₃ is given as:
Li (s) + KNO₃ (aq) → LiNO₃ + K
Thus the correct options are B and D.
To know more about chemical equation, visit;
https://brainly.com/question/19626681
#SPJ1
31. Why would a valence electron be easier to steal from a Francium atom than a Fluorine
atom? Give two reasons.
1.
2
Answers
Answer:
1. Because a Francium atom is deeper down the alkali metals, it is much easier to lose a valence electron.
2. A fluorine atom can easily gain a valence electron, but it could not easily lose a valence electron because it is one electron away from filling the outer shell
Explanation:
The answer is the explanation
Which example is a long-term environmental change?
O La Niña
O EI Nino
O climate change
O small asteroid impact
C
Answers
Which of the following statements about idea models is not true?
a.
Idea models depict a concept.
b.
Idea models are typically used to predict natural disasters.
c.
Idea models are typically in the form of a graph or equation.
d.
Idea models are used to show the relationship between variables.
Please select the best answer from the choices provided
A
B
C
D
Answers
Answer:
B - Idea models are not typically used to predict natural disasters.
arrange tha following substances in order of increasing boiling point and increasing solubility in water. -2-butanol ,-2-propanol,-2-methylpropane, -2-pentanol
Answers
Answer:
Here is the order of increasing boiling point and increasing solubility in water for the substances you listed:
-2-Methylpropane < -2-butanol < -2-pentanol < -2-propanol
Explanation:
-2-Methylpropane has the lowest boiling point and is the least soluble in water.
-2-butanol has a higher boiling point than -2-methylpropane, but lower than -2-pentanol and -2-propanol
-2-pentanol has a higher boiling point than -2-butanol and -2-methylpropane, but lower than -2-propanol
-2-propanol has the highest boiling point and is the most soluble in water.
Please note that this is a general trend and there can be variations depending on factors such as pressure, purity of the substance, and temperature.
Below is the titration curve of a 50.0 mL of 0.1 M triprotic acid H3A with 0.1 M NaOH(aq). The initial pH and the equivalence points are indicated in the curve.
Calculate pKa1, pKa2, and pKa3 for the triprotic acid H3A.

Answers
The first equivalence point, where half of the H3A has been neutralized, occurs at approximately pH 4.3. This corresponds to the point where H2A^- is formed; therefore, Ka1 can be calculated as follows:
Ka1 = [H2A^-][H3O^+]/[H3A] = 10^(-pH1)
where pH1 is the pH at the first equivalence point.
Substituting the given values, we get:
Ka1 = [H2A^-][H3O^+]/[H3A] = 10^(-4.3)
At the second equivalence point, where H2A^- has been completely neutralized and HA^2- is formed, the pH is approximately 8.5. Thus, Ka2 can be calculated as follows:
Ka2 = [HA^2-][H3O^+]/[H2A^-] = 10^(-pH2)
where pH2 is the pH at the second equivalence point.
Substituting the given values, we get:
Ka2 = [HA^2-][H3O^+]/[H2A^-] = 10^(-8.5)
Lastly, at the third equivalence point, where HA^2- has been completely neutralized and A^3- is formed, the pH is approximately 12.3. Therefore, Ka3 can be calculated as follows:
Ka3 = [A^3-][H3O^+]/[HA^2-] = 10^(-pH3)
where pH3 is the pH at the third equivalence point.
Substituting the given values, we get:
Ka3 = [A^3-][H3O^+]/[HA^2-] = 10^(-12.3)
Thus, the values of pKa1, pKa2, and pKa3 for the triprotic acid H3A are:
pKa1 = -log(Ka1) = -log(10^(-4.3)) = 4.3
pKa2 = -log(Ka2) = -log(10^(-8.5)) = 8.5
pKa3 = -log(Ka3) = -log(10^(-12.3)) = 12.3
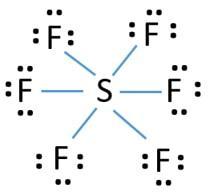
For each of the following two substances NOCl and SF6 answer the following questions:
Draw Lewis dot structure (include all dots necessary for each atom)
a.Identify the name of molecular geometry
b.Identify bond angles
c.Identify the molecular polarity of each substance
d.Calculate the formal charge of S atom in SF6
e.Calculate the formal charge of N atom in NOCl
Answers
There is zero formal charges on nitrogen in NOCl and sulfur in SF6.
The Lewis structure of NOCl is shown in the image attached. The NOCl molecule is bent or angular. The N - O - Cl bond angle in the molecule is about 113°.The molecule is polar, the formal charge on nitrogen is calculated as follows;
FC = 5 - 2 - 6/2 = 0
The Lewis structure of SF6 is shown in the image attached. The molecule is octahedral hence the bond angle in the molecule is 90°. The molecule is nonpolar because it is symmetrical. The formal charge on sulfur atom is zero.
Learn more: https://brainly.com/question/5624100

What are the two ways
that heat is measured?
Answers
Answer:
heat is mesured in calories and also joules
Explanation:
The density of ethanol is 0.789 g/cm^3. A flask holds 165.0 g of ethanol. What is the volume of the ethanol?
a. 4.78 x 10^-3 g
b. 130. g
c. 209 g
Answers
The volume of the ethanol that has a density of ethanol is 0.789 g/cm³ is 209.13cm³.
How to calculate volume?The volume of a substance can be calculated by dividing the mass of the substance by its density as follows:
Volume = mass ÷ density
According to this question, the density of ethanol is 0.789 g/cm³. A flask holds 165.0 g of ethanol and the volume can be calculated as follows:
Volume = 165.0g ÷ 0.789g/cm³
Volume = 209.13cm³
Therefore, the volume of the ethanol that has a density of ethanol is 0.789 g/cm³ is 209.13cm³.
Learn more about volume at: https://brainly.com/question/952755
#SPJ1
Which of the following VSEPR shapes describes the geometry of a
molecule with two atoms bonded to a third central atom with no lone pairs on the central atom?
A. linear
B. bent
C. trigonal planar
D. tetrahedral
Answers
The shape of the molecule with two atoms bonded to a third central atom with no lone pairs on the central atom is Linear.
Hence the correct option is (A)- linear
As given, the central atom has no lone pairs and two atoms bonded to the central atom.
Therefore the shape of the molecule is linear.
For example, let's take the example of CO₂. Here, the central atom is C and has four valence electrons. C uses 1 pair o electrons to form a double bond with each atom of O. Hence, C uses a total of 4 electrons to form a double bond with the O atom. No lone pair is left with carbon to create distortion in the molecule.
Therefore, the shape of the molecule will be linear.
So, the correct option is A.
To learn more about the shapes of molecules, visit: https://brainly.com/question/15651521
#SPJ9
A liquid ester used to flavour food is believed to be impure. What would be the best way of testing its purity?
Answers
Answer:
Filter it
Explanation:
The half life of a first order reaction is 36.8 seconds. How many seconds must the reaction proceed before the reaction is 72.5% completed?
Answers
Answer:
It would take approximately
64.0
s
given those data.
Explanation:
21.3
s
=
0.693
k
∴
k
≈
3.25
⋅
10
−
2
s
−
1
ln
(
1
8
)
=
−
3.25
⋅
10
−
2
s
−
1
⋅
t
∴
t
≈
64.0
s
Each equation I used is assuming this reaction's kinetics are of the first order, which is cited. The first equation is a simplified version for the half life of a first order reactant, and the second equation is the general equation for first order reactions in chemical kinetics.
A 1.0 L solution AgNO3(ag) of and Pb(NO3)2(aq) has a Agconcentration of 0.020 M and a Pb2+ concentration of
0.0010 M. A 0.0010 mol sample of K2SO4(s) is added to the solution. Based on the information in the table above,
which of the following will occur? (Assume that the volume change of the solution is negligible.)
No precipitate will form.
(B) Only Ag2SO4(s) will precipitate.
(C) Only PbSO4(s) will precipitate.
(D) Both Ag2SO4(s) and PbSO4(s) will precipitate.
Answers
With an Ag concentration of 0.020 M and a Pb2+ concentration of
0.0010 M, Only PbSO4(s) will precipitate,
Option C is correct
What will precipitate?Generally, the equation for the Chemical Reaction is mathematically given as
Ag2SO4--->2Ag++SO4^{-2}
Where
PbSO4--->Pb2+ +SO4^{-2}
Therefore
Ag2SO4=[0.02^2[0.001]]
Ag2SO4=4*10^{-7}
and
PbSO4=[0.001^2[0.001]]
PbSO4=4*10^{-6}
In conclusion,Since PbSO4>>PAg2SO4, Only PbSO4(s) will precipitate.
Read more about Chemical Reaction
https://brainly.com/question/11231920
c) Discuss precision and Accuracy as they relate to types of errors.
what is the answer
Answers
Precision relates to the consistency and reproducibility of measurements, while accuracy reflects how close measurements are to the true value.
Precision and accuracy are two important concepts in the context of errors in measurements. While they both pertain to the quality of data, they refer to different aspects.
Precision refers to the degree of consistency or reproducibility in a series of measurements. It reflects the scatter or spread of data points around the average value. If the measurements have low scatter and are tightly clustered, they are considered precise. On the other hand, if the measurements have a high scatter and are widely dispersed, they are considered imprecise.
Accuracy, on the other hand, refers to the closeness of measurements to the true or target value. It represents how well the measured values align with the actual value. Accuracy is achieved when measurements have a small systematic or constant error, which is the difference between the average measured value and the true value.
Errors in measurements can be classified into two types: random errors and systematic errors.
Random errors are associated with the inherent limitations of measurement instruments or fluctuations in the measurement process. They lead to imprecise data and affect the precision of measurements. Random errors can be reduced by repeating measurements and calculating the average to minimize the effect of individual errors.
Systematic errors, on the other hand, are caused by consistent biases or inaccuracies in the measurement process. They affect the accuracy of measurements and lead to a deviation from the true value. Systematic errors can arise from factors such as instrumental calibration issues, environmental conditions, or experimental techniques. These errors need to be identified and minimized to improve the accuracy of measurements.
In summary, precision refers to the degree of consistency or reproducibility of measurements, while accuracy refers to the closeness of measurements to the true value. Random errors affect precision, while systematic errors affect accuracy. To ensure high-quality measurements, both precision and accuracy need to be considered and appropriate techniques should be employed to minimize errors.
Know more about Precision here:
https://brainly.com/question/30461151
#SPJ8