during their developmental process, b and t lymphocytes undergo a selective process that specializes them for reacting only to one specific
Answers
Specializes them for reacting only to one specific antigen.
An antigen is a marker that tells your immune device whether or not something for your frame is harmful or no longer. Antigens are observed on viruses, microorganisms, tumors, and everyday cells of your frame.
The antigen is a substance that is capable of stimulating an immune reaction, specifically activating lymphocytes, which can be the frame's contamination-fighting white blood cells.
The antigen may additionally originate from within the frame ("self-protein") or from the external surroundings ("non-self"). The immune device identifies and attacks "non-self" outside antigens and usually does no longer react to self-protein due to the poor selection of T cells inside the thymus and B cells in the bone marrow.
Learn more about Antigen here:-https://brainly.com/question/14564657
#SPJ4
Related Questions
How many grams does 4.3 x 10^24 molecules of H2O weigh?
Answers
To get the mass for these molecules, we will need to do three steps:
1 - convert from number of molecules to number of moles.
2 - calculate the molar mass for H₂O.
3 - use it to convert from number of moles to mass.
To convert from number of molecules to number of moles, we need to divide the number of molecules by the Avogadro's number:
\(\begin{gathered} N_A\approx6.02\times10^{23}mol^{-1} \\ n_{H_2O}=\frac{N_{H_2O}}{N_A}=\frac{4.3\times10^{24}}{6.02\times10^{23}mol^{-1}}=0.71428\ldots\times10^1mol=7.1428\ldots mol \end{gathered}\)Now, to calculate the molar mass of H₂O, we need the molar masses of H and O, which we can get on a periodic table:
\(\begin{gathered} M_H=1.00794g\/mol \\ M_O=15.9994g\/mol \\ M_{H_2O}=2\cdot M_H+1\cdot M_O \\ M_{H_2O}=(2\cdot1.00794+1\cdot15.9994)g\/mol \\ M_{H_2O}=(2.01588+1\cdot15.9994)g\/mol \\ M_{H_2O}=18.01528g\/mol \end{gathered}\)Using it, we can convert the number of moles to mass:
\(\begin{gathered} M_{H_{2}O}=\frac{m_{H_2O}}{n_{H_{2}O}} \\ m_{H_2O}=n_{H_2O}\cdot M_{H_{2}O} \\ m_{H_2O}=7.1428\ldots mol\cdot18.01528g\/mol \\ m_{H_2O}=128.680\ldots g \end{gathered}\)Now, the proper way of approximating this value is to maintain the same number of significant figures as the number of molecules, because we used multiplications and divisions. It has two significant figure, so we need to approximate accourdingly:
\(m_{H_{2}O}\approx130g\)So, there is 128.680... grams or, approximately, 130 grams of water.
what is the frequency of radio
Answers
Question
Answer
Explanation
Radio Frequency Is one of the electromagnetic frequency waves that lie in the range stretching from below 3 kilohertz to around 300 gigahertz and which includes the frequencies used for communication signals (such as for radio and television broadcasting and cellular telephone and satellite transmissions)
I hope this helps
2 Chemistry questions
look at images below
20 points since there is 2 question
This will help me get the rest of my assignment done since I have no idea what to do.


Answers
The isotopes' number and the sum of the protons and neutrons are the same because the latter is equal to the mass number of an element.
What is mass number?Mass number of an element is the total number of protons and neutrons in an atomic nucleus.
According to this question, a table is presented where the isotope number of different elements equate the sum of the protons and neutrons in the atom of that element.
This is because the sum of the neutrons and protons in an atom is equal to the mass number of an element. This means that the isotope number is the mass number of each isotope.
Learn more about mass number at: https://brainly.com/question/18803094
#SPJ1
Complete the table by checking the correct column(s) for each description.
Description
Isotonic
Solution
Hypotonic
Solution
Hypertonic
Solution
7. A solution that has the same osmotic
concentration as a cell's cytoplasm
8. A solution that causes a cell to shrivel
9. A solution that causes a cell to swell
10. A solution that neither shrinks nor swells a cell
11. A solution in which there is more water outside
the cell than inside the cell
12. A solution that causes water to move out of a cell
Answers
Answer:
7. isotonic
8. hypertonic
9. hypotonic
10. isotonic
11. hypotonic
12. hypertonic
What is an orbital ? I need this ASAP :)

Answers
Ca2+ is an example of
a. cation
b.anion
c.ionic bond
d. ionic compound
Answers
A. Cation
36. (Higher) 2Mg + O2 → 2Mgo. Limiting reactant is Mg (4 mol). What is the amount of
product (in mol)? PLEASE HELP ME HOW TO WORK THESE OUT!!
Answers
Answer:
4 mol MgO
Explanation:
convert the limiting reactant to product by using dimensional analysis in order to see how many moles total can be created of the product
4 mol Mg x \(\frac{2 mol MgO}{2 mol Mg}\) = 4 mol MgO
Which ions produce similar colors in the flame tests?
Answers
Answer:
Two ions that produced similar colors in the flame test were Ca+2 and Sr+2. 3.
Explanation:
The colors are produced when an electron jumps to a higher level and then jump back down.
Ba2+ and Cu2+ and Sr2+ and Li+ were the pair with the similar color. Sr and Li displayed red colors, while Ba and Cu had mild greenish yellowish hues.
Why do some ions in the flame test generate colors that are similar?
The precise sizes of the potential energy jumps differ from metal to metal. As a result, the flame color of each metal will differ due to its unique spectral line pattern. The movement of the electrons in the metal ions contained in the compounds results in the hues of the flame.
The energy released by each electron when it returns to its initial condition determines the hue of the light that is produced.
To learn more about ions in the flame refer to:
https://brainly.com/question/28715571
#SPJ2
Whose discovery led to the discovery of the proton?
A. Dalton
B. Rutherford
C. Aristotle
D. Bohr
Answers
A mixture of calcium chloride and calcium trioxocarbonate(IV) In water can be separated by which separating technique
Answers
Answer:
Filtration
Explanation:
6. Which specific processes in the rock cycle occur beneath the Earth's surface?
Support your answer.
Answers
Answer:
Under the earth's surface, rocks melt, metamorphize, and crystalize.
Explanation:
Metamorphic and Igneous rocks are basically dependant on the heat/pressure of the environment under the surface :) Melting, metamorphosing and crystallization all occur under earth's surface.
The specific processes in the rock cycle that occur beneath the Earth surface are :
Melting crystallizationmetamorphizationUnder the Earth surface the specific rock cycle processes that leads to the formation of the different types of rocks includes; Melting, metamorphization and rock crystallization.
Metamorphic rocks are formed from other rocks ( sedimentary and igneous rocks) due to the change in temperature and this process is metamorphization. while igneous rocks are formed from molten rocks caused by the melting process. Sedimentary rocks are formed by the deposition of sediments from weathering of existing rocks this is the crystallization process.
Hence the specific process in the rock cycle that occur beneath the Earth's surface are : Melting, metamorphosing and crystallization.
Learn more : https://brainly.com/question/22340852

clarify the following expression s
a.atomic number
b.mass number
c.nucleus
Answers
Answer:
Atomic number of a chemical element is the number of protons found in the nucleus of every atom of that element.
The mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons in an atomic nucleus.
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom.
0.75 kg to milligrams
Answers
Answer:
750000
Explanation:
multiply the mass value by 1e+6
Answer:
750000 mg is ur answer My G
Explanation:
No Explanation Needed Ig
explain in details how technetium_90m is generated?
Q7. Radio Isotopes in Health Care Explain how technetium-99m is generated.
Answers
Technetium-99m is a radioisotope that is widely used in nuclear medicine for various imaging studies. It is usually produced through a process called generator system from the decay of its parent isotope, Molybdenum-99 (Mo-99).
How technetium-99m is generated:Technetium-99m is generated by a process called a generator system from the decay of its parent isotope, Molybdenum-99 (Mo-99). This generator system is essentially a column packed with a gel-like substance, which is usually made of alumina, silica, or another material. The column contains Mo-99, which is produced in a nuclear reactor, and its daughter isotope Technetium-99m (Tc-99m).The Mo-99 decays into Tc-99m by beta decay, emitting a beta particle and a neutrino.
As a result, Tc-99m is separated from Mo-99 by using a saline solution or another eluant to flush the column. The Tc-99m-containing eluant is then used for imaging studies.There are several advantages to using Tc-99m for imaging studies. It has a short half-life of only six hours, which means that it does not stay in the body for a long time and is eliminated quickly. This makes it safer for patients than isotopes with longer half-lives. Additionally, Tc-99m emits gamma rays, which can be detected by imaging equipment such as gamma cameras. This allows for high-quality imaging studies that can help diagnose a wide range of medical conditions.
Learn more about Technetium-99m: https://brainly.com/question/20064537
#SPJ11
can you help again
will be the brainiest

Answers
Answer:
B
Explanation:
How do I solve for a and b using the Van Der Waals equation using only the given values: P= 1 atm, V= 1.310 L, and T= 160 K
Answers
Answer:
\(a = \frac{24.79078- 1.7161b}{1.310 - b}\)
\(b = 1.310 - \frac{22.5427}{a - 1.7161}\)
Explanation:
Given
\(P = 1\ atm\)
\(V = 1.310\ L\)
\(T =160\ K\)
Required
Solve for a and b
Van Der Waals equation is:
\(P = \frac{RT}{V - b} - \frac{a}{V^2}\)
Substitute values for P, V and T, we have:
\(1 = \frac{R*160}{1.310 - b} - \frac{a}{1.310^2}\)
R is a constant and the value is:
\(R = 0.0821\)
So, the equation becomes:
\(1 = \frac{0.0821*160}{1.310 - b} - \frac{a}{1.310^2}\)
Simplify the expression
\(1 = \frac{13.136}{1.310 - b} - \frac{a}{1.7161}\) ----- (a)
Solving for (a):
\(1 + \frac{13.136}{1.310 - b} = \frac{a}{1.7161}\)
Multiply both sides by 1.7161
\(a = [1 + \frac{13.136}{1.310 - b}] * 1.7161\)
Take LCM
\(a = [\frac{1.310 - b+13.136}{1.310 - b}] * 1.7161\)
Evaluate like terms
\(a = [\frac{14.446- b}{1.310 - b}] * 1.7161\)
Open bracket
\(a = [\frac{24.79078- 1.7161b}{1.310 - b}\)
Solving for (b), we have:
\(1 + \frac{13.136}{1.310 - b} = \frac{a}{1.7161}\)
Subtract 1 from both sides
\(\frac{13.136}{1.310 - b} = \frac{a}{1.7161}-1\)
Take LCM
\(\frac{13.136}{1.310 - b} = \frac{a-1.7161}{1.7161}\)
Inverse both sides
\(\frac{1.310 - b}{13.136} = \frac{1.7161}{a - 1.7161}\)
Multiply both sides by 13.136
\(1.310 - b = 13.136 * \frac{1.7161}{a - 1.7161}\)
\(1.310 - b = \frac{22.5427}{a - 1.7161}\)
Collect like terms
\(b = 1.310 - \frac{22.5427}{a - 1.7161}\)
Now suppose a reaction vessel is filled with 0.0406 atm of nitrogen (N_2) and 5.97 atm of ammonia (NH_3) at 1126. Degree C. Answer the following question this system: Under these conditions, will the pressure of N_2 tend to rise or fall? rise fall Is it possible to reverse this tendency by adding H_2? In other words, if you said the pressure of N_2 will tend to rise, can that be changed to a tendency to fall adding H_2? Similarly, if you said the pressure of N_2 will tend to fall, can that be changed to a tendency to rise by adding H_2? Yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of H_2 needed to reverse it. Round your answer to 2 significant digits. atm
Answers
The pressure of \(N_{2}\) will rise under the given conditions. And, Yes, it is possible to reverse this tendency by adding \(H_{2}\). The minimum pressure of H2 required to reverse the tendency is 0.01 atm.
The reaction involved is: \(N_{2}\)(g) + 3\(H_{2}\)(g) ⇌ 2\(NH_{3}\)(g) Hence, when \(H_{2}\) is added to the above system, the \(N_{2}\) and \(H_{2}\) will react to produce \(NH_{3}\). This reaction will reduce the amount of \(N_{2}\) present in the system, causing the pressure of \(N_{2}\) to decrease. Therefore, by adding \(H_{2}\) , we can change the tendency of \(N_{2}\) pressure from rise to fall.To calculate the minimum pressure of \(H_{2}\) required to reverse the tendency, we have to use the equilibrium constant, Kp. The expression for Kp for the above reaction is: Kp =( \(NH_{3}\)) / p(\(N_{2}\)) p3( \(H_{2}\) )
At equilibrium, Kp = 1.7 × 104 at 1126 °C.Now, we will solve for the minimum pressure of \(H_{2}\) needed to reverse the tendency. Let's assume that the pressure of \(N_{2}\) has increased by x atm. Therefore, the new pressure of \(N_{2}\) will be (0.0406 + x) atm. At equilibrium, we have:
p2(\(NH_{3}\) ) / p(\(N_{2}\)) p3( \(H_{2}\) ) = 1.7 × 104
On substituting the given values and simplifying, we get:
p2(\(NH_{3}\)) / p(N2) = 6.39 × 10-5
Now, p2(\(NH_{3}\)) = 5.97 atm, and p(\(N_{2}\)) = (0.0406 + x) atm.
On substituting these values, we get:5.97 / (0.0406 + x) = 6.39 × 10-5
Solving for x, we get:x = 0.00579 atm ≈ 0.01 atm (rounded to 2 significant digits)Therefore, the minimum pressure of \(H_{2}\) required to reverse the tendency is 0.01 atm.
More on equilibrium: https://brainly.com/question/30188799
#SPJ11
hydrogen bonds can form between hydrogen and any non-metal. true or false
Answers
The statement "hydrogen bonds can form between hydrogen and any non-metal" is FALSE.
Hydrogen bonds are the type of chemical bonds formed between a partially positively charged hydrogen atom of one molecule and a partially negatively charged electronegative atom (oxygen, nitrogen, or fluorine) of a different molecule.
The bond is between the hydrogen atom and the electronegative atom rather than between the two atoms involved. Because of the fact that only three atoms - oxygen, nitrogen, and fluorine - are the only ones electronegative enough to form hydrogen bonds with hydrogen, the statement above is false because hydrogen bonds only form between hydrogen and nitrogen, oxygen, or fluorine.
To know more about hydrogen bonds refer to:
https://brainly.com/question/11679211
#SPJ11
why is it not possible to test for ammonia as evidence of nitrate reduction to ammonia in the nitrate broth culture
Answers
Ammonia as proof that nitrate was converted to ammonia in the cultivation of nitrate broth Because amino acids are produced during protein synthesis, amino acid metabolism will always exist.
Nitrifying microorganisms in the soil change ammonia into nitrates. Nitrates are taken up by plants from the soil and used to assemble proteins. Animals may consume the plant, and its biomass may be used to produce animal protein.
The process of nitrate (NO3) being used as an electron acceptor for respiration microbes results in dissimilatory nitrate reduction to ammonium (DNRA), also known as nitrate/nitrite ammonification.
Read more about nitrate reduction at
https://brainly.com/question/29316155
#SPJ4
if you are correct you get a brainless
Tarek would like to germinate pumpkin seeds for his science project. He wraps the seeds in a dry paper towel and places them on the windowsill. Tarek waits, but the seeds will not grow. What conditions should Tarek have used to germinate the seeds? (2 points)
a
The paper towel should be wet, and the seeds should be placed in a dark place.
b
The dry paper towel should be placed in a pot filled with soil.
c
The paper towel should be dry and transparent.
d
The seeds should be placed in a dark place.
Answers
Answer:
A.
Explanation:
❤️
Answer: it’s A. The paper towel should be wet, and the seeds should be placed in a dark place.
Explanation: I hope this helps!
I NEED THIS ASAP!! Select all that apply.
Air pollution particles may be removed by _____.
oceans
natural cycles
soil
rain
snow
wind
Answers
Answer:
Natural Cycles
Explanation:
Most sulfur oxides are produced by burning: coal
Air pollution particles may be removed by _____. rain snow wind natural cycles
The three primary sources of air pollution are _____. factories cars electric power plants
The two basic types of air pollutants are _____. gases particulates
May I get brainliest please?
If I'm wrong sorry.
calculate the maximum number of grams of nh3 that can be produced by the reaction of 2.00 g of n2 with 3.00 g h2.
Answers
The maximum number of grams of NH3 that can be produced by the reaction of 2.00 g of N2 with 3.00 g of H2 is 4.00 g.
This is because of the stoichiometric proportions of the reaction: 2 moles of N2 and 3 moles of H2 will produce 2 moles of NH3. According to the ideal gas law, 2.00 g of N2 and 3.00 g of H2 will produce 4.00 g of NH3.
The stoichiometry of the reaction can be calculated using the mole ratio of the reactants. Two moles of N2 are needed for every three moles of H2 in order to produce two moles of NH3.
This means that the ratio of N2 to H2 in the reaction is 2:3. To convert the grams of each reactant into moles, the molecular weight of each reactant is used.
N2 has a molecular weight of 28.02 g/mol and H2 has a molecular weight of 2.02 g/mol.
Once the mole ratios and molecular weights have been determined, the moles of each reactant can be calculated. 2.00 g of N2 is equal to 0.0714 mol, and 3.00 g of H2 is equal to 1.48 mol.
Using the mole ratios, the number of moles of NH3 that can be produced can be calculated. In this case, the maximum number of moles of NH3 is 0.0714 mol, since this is the limiting reactant.
The number of grams of NH3 produced can then be calculated using the molecular weight of NH3, which is 17.04 g/mol. 0.0714 mol of NH3 is equal to 1.22 g of NH3.
In conclusion, the maximum number of grams of NH3 that can be produced by the reaction of 2.00 g of N2 with 3.00 g of H2 is 4.00 g. This is due to the stoichiometric proportions of the reaction and the ideal gas law.
to know more about reaction referhere:
https://brainly.com/question/27948961#
#SPJ11
What volume will be occupied by 40.2 g of neon gas at STP?
Answers
Answer:
44.8 L
Explanation:
1 mole of a gas at s.t.p. occupies a volume of 22.4 L.
Molar mass of Neon is 20.1 g/mol
Number of moles of neon present in 40.2 g = Mass/molar mass
Number of moles = 40.2 / 20.1 = 2 moles.
Therefore, 2 moles of neon gas will occupy 2 * 22.4 L = 44.8 L
44.8 L
Molar mass of Neon is 20.1 g/mol
Number of moles of neon present in 40.2 g =\(Mass/molar mass\)
\(Number of moles = 40.2 / 20.1 \\= 2 moles.\)
At STP:-
Gases have a volume of 22.4L/mol.
Therefore, 2 moles of neon gas will occupy the volume is as follows:-
\(2 mol\times22.4 L/mol = 44.8 L\)
So, the volume is 44.8 L.
To know more about:-
brainly.com/question/24050436
Which sample contains the greatest number of molecules?
A 4g of hydrogen
B 18 g of water
C 24 dm³ of oxygen
D 66 g of carbon dioxide
And why is the answer that way?
Answers
Answer:
4g of hydrogen
Explanation:
4g of hydrogen (molar mass 2) corresponds to 2 moles of molecules or 4N number of atoms where N is the Avogadro's number (6.023×10
23)
Hi please help me!!
Propane (C3H8) burns in oxygen to form CO2 and H2O according to the following equation. How many grams of O2 are required to burn 2.56 x 1022 propane molecules?
(This chemical equation is not balanced. You need to balance this chemical equation first before calculation)
C3H8 + O2 --> CO2 + H2O
Answers
Answer:
The balanced equation for the combustion of propane is:
C3H8 + 5O2 --> 3CO2 + 4H2O
So for every propane molecule, we need 5 oxygen molecules.
To calculate the number of oxygen molecules required to burn 2.56 x 10^22 propane molecules, we need to multiply the number of propane molecules by the ratio of oxygen molecules to propane molecules.
Ratio of O2 to C3H8 = 5:1
Number of O2 molecules required = (5/1) x 2.56 x 10^22 = 1.28 x 10^23
Now we can convert the number of oxygen molecules to grams using the molar mass of oxygen.
1 mole of O2 = 32 g
1.28 x 10^23 molecules of O2 = (1.28 x 10^23 / 6.022 x 10^23) moles of O2
Mass of O2 = (1.28 x 10^23 / 6.022 x 10^23) x 32 g
Mass of O2 = 6.82 grams
So, 6.82 grams of O2 are required to burn 2.56 x 10^22 propane molecules.
What is the mass in grams of 10 moles of ammonia, NH3?
A.
?
1.7 grams
B.
?
27 grams
C. ?
170 grams
D
?
0.587 grams
Answers
Answer:
C. 170 g.
Explanation:
multiply given moles by the molar mass of ammonia.
what state of matter is water
Answers
Answer:
water is liquid state of matter
Which of the following is a characteristic property of ionic compounds? A.They have low melting points. B.They form hard, brittle crystals. C.They do not form crystals. D.They have low boiling points.
Answers
Answer:
the answer is d
Explanation:
i just did it
The characteristic property of ionic compounds is B. They form hard, brittle crystals.
Ionic bond is a chemical bond formed as the result of transfer of electrons from one atom to another.
Ionic bonds are held by strong electrostatic force. Properties of ionic bonds are:
There form crystals.they have high boiling and melting points.The are soluble in water and insoluble in solvents.They conduct electricity when dissolved in water.Find out more at: https://brainly.com/question/11148793
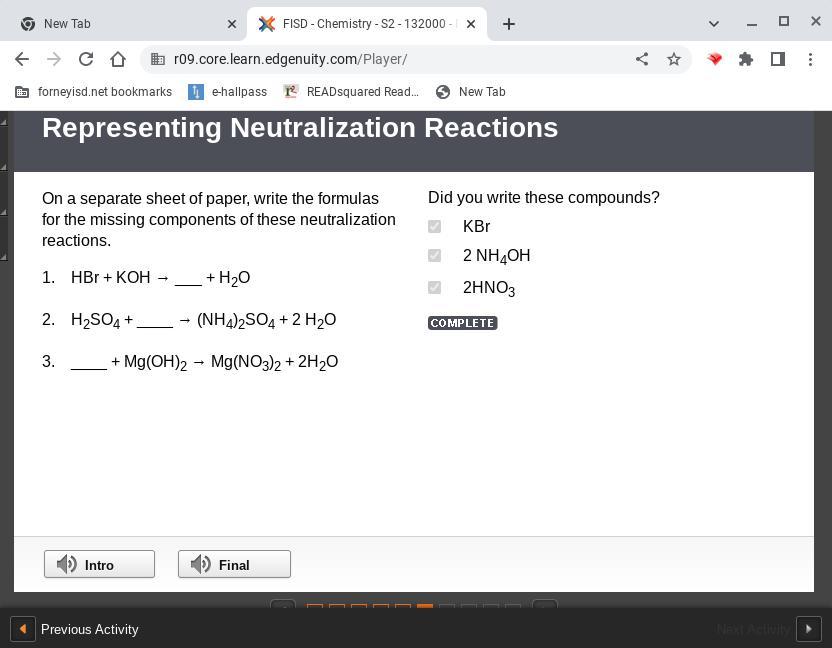
On a separate sheet of paper, write the formulas for the missing components of these neutralization reactions. 1. hbr koh → ___ h2o 2. h2so4 ____ → (nh4)2so4 2 h2o 3. ____ mg(oh)2 → mg(no3)2 2h2o
Answers
The missing products of the neutralization reactions are;
1) KBr
2) NH3
3) HNO3
What is a neutralization reaction?A neutralization reaction is a reaction that occurs between an acid and a base to yield salt and water only.
The missing products of the neutralization reactions are;
1) KBr
2) NH3
3) HNO3
Learn more about neutralization:https://brainly.com/question/15395418
#SPJ4
Answer:
KBr
2 NH4OH
2 HNO3
Explanation:
At 9°C a gas has a volume of 6.17 L. What is its volume when the gas is at standard temperature?
Answers
Answer:
V₂ = 5.97 L
Explanation:
Given data:
Initial temperature = 9°C (9+273 = 282 K)
Initial volume of gas = 6.17 L
Final volume of gas = ?
Final temperature = standard = 273 K
Solution:
Formula:
The Charles Law will be apply to solve the given problem.
According to this law, 'the volume of given amount of a gas is directly proportional to its temperature at constant number of moles and pressure'
Mathematical expression:
V₁/T₁ = V₂/T₂
V₁ = Initial volume
T₁ = Initial temperature
V₂ = Final volume
T₂ = Final temperature
Now we will put the values in formula.
V₁/T₁ = V₂/T₂
V₂ = V₁T₂/T₁
V₂ = 6.17 L × 273K / 282 k
V₂ = 1684.41 L.K / 282 K
V₂ = 5.97 L