draw the structure of the product formed when the given compound is heated in aqueous base. the formula for the product isC8H12O
Answers
The structure of the product with the formula C8H12O is a cyclic ketone with a six-membered ring and two double bonds
To draw the structure of the product formed when the given compound is heated in aqueous base with the formula C8H12O, follow these steps:
Count the number of carbons, hydrogens, and oxygens in the formula: C8H12O has 8 carbons, 12 hydrogens, and 1 oxygenDetermine the degree of unsaturation: Degree of Unsaturation = (2 * Carbon atoms + 2 - Hydrogen atoms + Oxygen atoms) / 2. For C8H12O, it is (2 * 8 + 2 - 12 + 1) / 2 = 3. This indicates that there are 3 double bonds or rings in the molecule.Begin drawing the structure by connecting 8 carbon atoms in a linear fashion.Add double bonds or rings to satisfy the degree of unsaturation. For example, you can create a six-membered ring by connecting carbons 1 and 6, and add a double bond between carbons 2 and 3 and another double bond between carbons 4 and 5.Add the oxygen atom to the structure. Since there is only one oxygen atom, it can be added as a functional group. In this case, it can be added as a ketone group at the carbon 3 position.Finally, fill in the remaining hydrogen atoms to complete the structure. There should be two hydrogens on carbons 1, 6, 7, and 8, and one hydrogen on carbons 2 and 5.The structure of the product formed when the given compound is heated in aqueous base with the formula C8H12O is a cyclic ketone with a six-membered ring and two double bonds.
Learn more about C8H12O: brainly.com/question/30008257
#SPJ11
Related Questions
if the ph is less than the pka, the molecule is mostly [ select ] . if the ph is greater than the pka, the molecule is mostly [ select ] . if the ph equals the pka, the molecule is equal parts protonated and deprotonated.
Answers
If the ph is less than the pka, the molecule is mostly protonated. if the ph is greater than the pka, the molecule is mostly deprotonated. if the ph equals the pka, the molecule is equal parts protonated and deprotonated.
The pH of a molecule determines whether it is mostly protonated or deprotonated. If the pH is less than the pKa, the molecule is mostly protonated. If the pH is greater than the pKa, the molecule is mostly deprotonated. If the pH equals the pKa, the molecule is equal parts protonated and deprotonated.
The relationship between pH and pKa is as follows: pH pKa: deprotonated form is favored. pH = pKa: equal amounts of protonated and deprotonated forms. In essence, the pH of a solution impacts how a molecule behaves in terms of whether it's protonated or deprotonated.
A pH that is lower than the pKa of the molecule indicates that the protonated form is favored. A pH that is higher than the pKa of the molecule suggests that the deprotonated form is favored. If the pH and the pKa of a molecule are equal, the molecule is equal parts protonated and deprotonated.
For more such questions on molecule, click on:
https://brainly.com/question/30375112
#SPJ11
Do tanning beds have nuclear fission or fusion?
Answers
Answer:
Neither? If it must be one then fission.
Explanation:
I actually didn't know they had fission, but I can absolutely gaurantee that they don't use fusion, nuclear fusion is a process that fuses hydrogen atoms together and releases a massive amount of energy, it used in hydrogen bombs and by our sun. It's a process that needs a massive amount of energy to kickstart and there's no way that technology would fit in a tanning bed. If I'm wrong then please tell me.
Hope this is a good answer.
what is the importance of polar covalent and hydrogen bonds in the structure of water?
Answers
Answer:
Water is a remarkable substance, and its unique properties are largely due to the presence of polar covalent bonds and hydrogen bonds in its structure. These characteristics play a crucial role in the physical and chemical properties of water, making it essential for life as we know it.
Explanation:
The polar covalent bonds in water arise from the unequal sharing of electrons between oxygen and hydrogen atoms. This results in the oxygen atom having a partial negative charge (δ-) and the hydrogen atoms having partial positive charges (δ+). These charges create polarity within the water molecule, leading to the formation of hydrogen bonds.
Hydrogen bonds occur when the partially positive hydrogen atom of one water molecule is attracted to the partially negative oxygen atom of another water molecule. These hydrogen bonds are relatively weak individually, but when present in large numbers, they contribute to the cohesion, surface tension, and high boiling point of water.
The importance of these bonds is manifold. The cohesion between water molecules due to hydrogen bonding enables water to form droplets, have a high surface tension, and flow freely, facilitating transport within organisms and in the environment. Additionally, hydrogen bonding leads to the high specific heat capacity and heat of vaporization of water, making it an effective regulator of temperature in living organisms and ensuring stable environmental conditions.
Furthermore, hydrogen bonds play a crucial role in the unique properties of water as a solvent. The polar nature of water allows it to dissolve a wide range of substances, including ionic compounds and polar molecules, facilitating various biological processes such as nutrient transport and chemical reactions in cells.
How is Earth's surface most likely to change when a river flows across a steep landscape over time?
It will form into a mountain due to deposition.
Physical weathering will create a channel with curvy loops.
A valley will be carved out due to erosion.
A lake will form due to chemical weathering.
Answers
Answer:c
Explanation:
A valley will be carved out due to erosion.
Answer:
it was c
Explanation:
the dihydrogenphosphate ion, h2po4? is amphiprotic. in which of the following reactions is this ion serving as a base?
Answers
A substance that can donate a proton (H+) is known as an acid, while one that can accept a proton is known as a base.
The reaction of the dihydrogenphosphate ion with water indicates that it is an amphiprotic substance:H2PO4- + H2O ⇌ H3O+ + HPO42-
The following reaction shows that the dihydrogenphosphate ion is serving as a base:H2PO4- + NH4+ → HPO42- + NH4+H+.
Summary: Hence, the dihydrogenphosphate ion serves as a base in the reaction given as H2PO4- + NH4+ → HPO42- + NH4+H+.
Learn more about acid click here:
https://brainly.com/question/25148363
#SPJ11
my chem test is tomorrow!! please help

Answers
Answer:
The pH of the pure water is not always neutral but actually sometimes it can be slightly acidic but not of the same extent or value as HCl obviously. However ,it is enough to result to a color change in the presence of phenolphthalein.
Explanation:
I will try to be brief and spare the boring details .Basically ,this is caused by the reaction of water and CO2 that is naturally present in the atmosphere to produce a weak acid known as carbonic acid.
~Hope this helps:)
Is ceramic a good insulator
Answers
GIVING BRAINLIEST AND THANKS!
Can anyone help with this worksheet?
There are three mole equalities. They are: 1 mol = 6.02 x 1023 particles 1 mol = molar mass in g (periodic table) 1 mol = 22.4 L for a gas at STP
How many moles are there in 2750 ml of Hydrogen?
How many atoms are there in 27.8 L of Oxygen.?
How many atoms are in 0.62 mole of water?
Calculate the number of moles of hydrogen are in 1.7 x 1022atoms
Calculate the number of atoms in 2500 L of water
Calculate the mass of 2.5mol 2NH3
Given the following balanced chemical equation:
C5H12+8O2→5CO2+6H2O
How many moles of H2O can be formed if 0.0652 mol of C5H12 were to react?
Balance the following unbalanced equation and determine how many moles of H2O are produced when 1.65 mol of NH3 react:
NH3+O2→N2+H2O
8. How many moles of oxygen react with hydrogen to produce 27.6 mol of H2O
? Unbalanced: H2 + O2 → H2O balanced equation and solve.
9. If we have 3.59 mol of Fe2O3 , how many grams of SO3 can react with it?
Fe2O3 + 3SO3 —------> Fe2(SO4)3
1. How many moles of magnesium is 3.01 x 1022 atoms of magnesium?
2. How many molecules are there in 4.00 moles of glucose, C6H12O6
?
3. How many moles are 1.20 x 1025 atoms of phosphorous?
4. How many atoms are in 0.750 moles of zinc?
5. How many molecules are in 0.400 moles of N2O5?
Mole-Mass Conversions
1. How many moles in 28 grams of CO2?
2. What is the mass of 5 moles of Fe2O3 ?
3. Find the number of moles of argon in 452 g of argon.
4. Find the grams in 1.26 x 10-4 mol of HC2H3O2.
5. Find the mass in 2.6 mol of lithium bromide.
Mole-Volume Conversions
1. Determine the volume, in liters, occupied by 0.030 moles of a gas at STP.
2. How many moles of argon atoms are present in 11.2 L of argon gas at STP?
3. What is the volume of 0.05 mol of neon gas at STP?
Answers
There are 0.123 moles of Hydrogen in 2750 mL of Hydrogen; There are 1.51 x 10²⁴ Oxygen atoms in 27.8 L of Oxygen ; There are 1.11 x 10²⁴ atoms in 0.62 mole of water; There are 0.282 moles of hydrogen in 1.7 x 10²² atoms : There are approximately 2.02 x 10²⁶ atoms in 2500 L water. mass of 2.5 mol of NH3 is 42.57 g.
What is a chemical equation?Symbolic representation of chemical reaction in form of symbols and chemical formulas is called balanced chemical equation.
1 mol H2 = 22.4 L H2
x mol H2 = 2.75 L H2
x = 2.75 L H2 / 22.4 L H2
x = 0.123 mol H2
Therefore, there are 0.123 moles of Hydrogen in 2750 mL of Hydrogen.
1 mol O2 = 22.4 L O2
x mol O2 = 27.8 L O2
x = 27.8 L O2 / 22.4 L O2
x = 1.24 mol O2
1 mol O2 = 6.02 x 10²³ O2 molecules
1.24 mol O2 = 1.24 x 6.02 x 10²³ O2 molecules
1.24 mol O2 = 7.53 x 10²³ O2 molecules
7.53 x 10²³ O2 molecules x 2 atoms O per molecule = 1.51 x 10²⁴ Oxygen atoms
Therefore, there are 1.51 x 10²⁴ Oxygen atoms in 27.8 L of Oxygen.
As 1 mole H2O is 6.02 x 10²³ H2O molecules
and 1 H2O molecule=2 H atoms+1 O atom= 3 atoms
0.62 mol H2O x 6.02 x 10²³ H2O molecules/mol x 3 atoms/H2O molecule = 1.11 x 10²⁴ atoms
Therefore, there are 1.11 x 10²⁴ atoms in 0.62 mole of water.
1 mol H2= 6.02 x 10²³ H2 molecules
1.7 x 10²² H2 molecules / 6.02 x 10²³ H2 molecules per mole = x moles H2
x =0.282 mol H2
Therefore, there are 0.282 moles of hydrogen in 1.7 x 10²² atoms.
2500 L of water / 22.4 L/mol = 111.6 mol of water
111.6 mol of water x 6.02 x 10²³ molecules/mol = 6.72 x 10²⁵ molecules of water
6.72 x 10²⁵ molecules of water x 3 atoms/molecule = 2.02 x 10²⁶ atoms of hydrogen and oxygen in 2500 L water.
Therefore, there are approximately 2.02 x 10²⁶ atoms in 2500 L water.
As, mass is number of moles x molar mass
= 2.5 mol NH3 x 17.03 g/mol NH3
mass = 42.57 g
Therefore, mass of 2.5 mol of NH3 is 42.57 g.
C5H12 + 8O2 → 5CO2 + 6H2O
0.0652 mol C5H12 x 6 mol H2O/1 mol C5H12 = 0.3912 mol H2O
And therefore, 0.3912 mol H2O is formed if 0.0652 mol of C5H12 react.
Balance equation: NH3 + 2O2 → N2 + 3H2O
1.65 mol NH3 x 3 mol H2O/1 mol NH3 = 4.95 mol H2O
Therefore, 4.95 mol of H2O are produced when 1.65 mol of NH3 reacts.
From balanced equation: 2H2 + O2 → 2H2O
So, 27.6 mol H2Ox 1 mol O2/2 mol H2O=13.8 mol O2
Therefore, 13.8 mol of O2 react with hydrogen to produce 27.6 mol of H2O.
Using balanced equation: Fe2O3 + 3SO3 → Fe2(SO4)3
3.59 mol Fe2O3 x 3 mol SO3/1 mol Fe2O3 = 10.77 mol SO3
10.77 mol SO3 x 80.06 g/mol = 862.6 g SO3
Therefore, 862.6 g of SO3 can react with 3.59 mol of Fe2O3.
3.01 x 10²² atoms of Mg / 6.02 x 10^23 atoms/mol = 0.050 mol of Mg
Therefore, 3.01 x 10^22 atoms of Mg is equal to 0.050 mol of Mg.
1 mol of glucose (C6H12O6) = 6.02 x 10²³ molecules
Therefore: 4.00 mol of glucose x 6.02 x 10²³ molecules/mol = 2.41 x 10²⁴ molecules of glucose
Therefore, there are 2.41 x 10²⁴ molecules in 4.00 moles of glucose.
1 mol of phosphorous = 6.02 x 10²³ atoms
Therefore: 1.20 x 10²⁵ atoms of phosphorous / 6.02 x 10²³ atoms/mol = 19.9 mol of phosphorous
Therefore, 1.20 x 10²⁵ atoms of phosphorous is equal to 19.9 mol of phosphorous.
1 mol of zinc = 6.02 x 10²³ atoms
Therefore: 0.750 mol of zinc x 6.02 x 10²³ atoms/mol = 4.52 x 10²³ atoms of zinc
Therefore, there are 4.52 x 10²³ atoms in 0.750 moles of zinc.
1 mol of N2O5 = 6.02 x 10²³ molecules
Therefore: 0.400 mol of N2O5 x 6.02 x 10²³ molecules/mol = 2.41 x 10²³ molecules of N2O5
Therefore, there are 2.41 x 10²³ molecules in 0.400 moles of N2O5.
Molar mass of CO2 is 12.01 + 2(16.00) = 44.01 g/mol.
moles = mass/molar mass = 28 g/44.01 g/mol = 0.636 mol
Therefore, there are 0.636 moles in 28 grams of CO2.
Molar mass of Fe2O3 is 2(55.85) + 3(16.00) = 159.69 g/mol.
mass = moles x molar mass = 5 mol x 159.69 g/mol = 798.45 g
Therefore, mass of 5 moles of Fe2O3 is 798.45 grams.
Molar mass of Ar is 39.95 g/mol.
moles = mass/molar mass = 452 g/39.95 g/mol = 11.3 mol
Therefore, there are 11.3 moles of Ar in 452 grams of Ar.
Molar mass of HC2H3O2 is 1(1.01) + 2(12.01) + 2(1.01) + 2(16.00) = 60.05 g/mol.
mass = moles x molar mass = 1.26 x 10⁻⁴ mol x 60.05 g/mol = 0.00756 g
Therefore, there are 0.00756 grams in 1.26 x 10⁻⁴ mol of HC2H3O2.
Molar mass of LiBr is 6.94 + 79.90 = 86.84 g/mol.
mass = moles x molar mass = 2.6 mol x 86.84 g/mol = 225.784 g
Therefore, mass in 2.6 mol of LiBr is 225.784 grams.
volume = moles x 22.4 L/mol = 0.030 mol x 22.4 L/mol = 0.672 L
Therefore, 0.030 moles of a gas at STP occupies volume of 0.672 liters.
moles = volume/22.4 L/mol = 11.2 L/22.4 L/mol = 0.5 mol
Therefore, 0.5 moles of argon atoms present in 11.2 L of argon gas at STP.
Therefore, to find volume of 0.05 mol of neon gas at STP:
volume = moles x 22.4 L/mol = 0.05 mol x 22.4 L/mol = 1.12 L
Therefore, 0.05 mol of neon gas at STP occupies volume of 1.12 liters.
To know more about balanced chemical equation, refer
https://brainly.com/question/11904811
#SPJ1
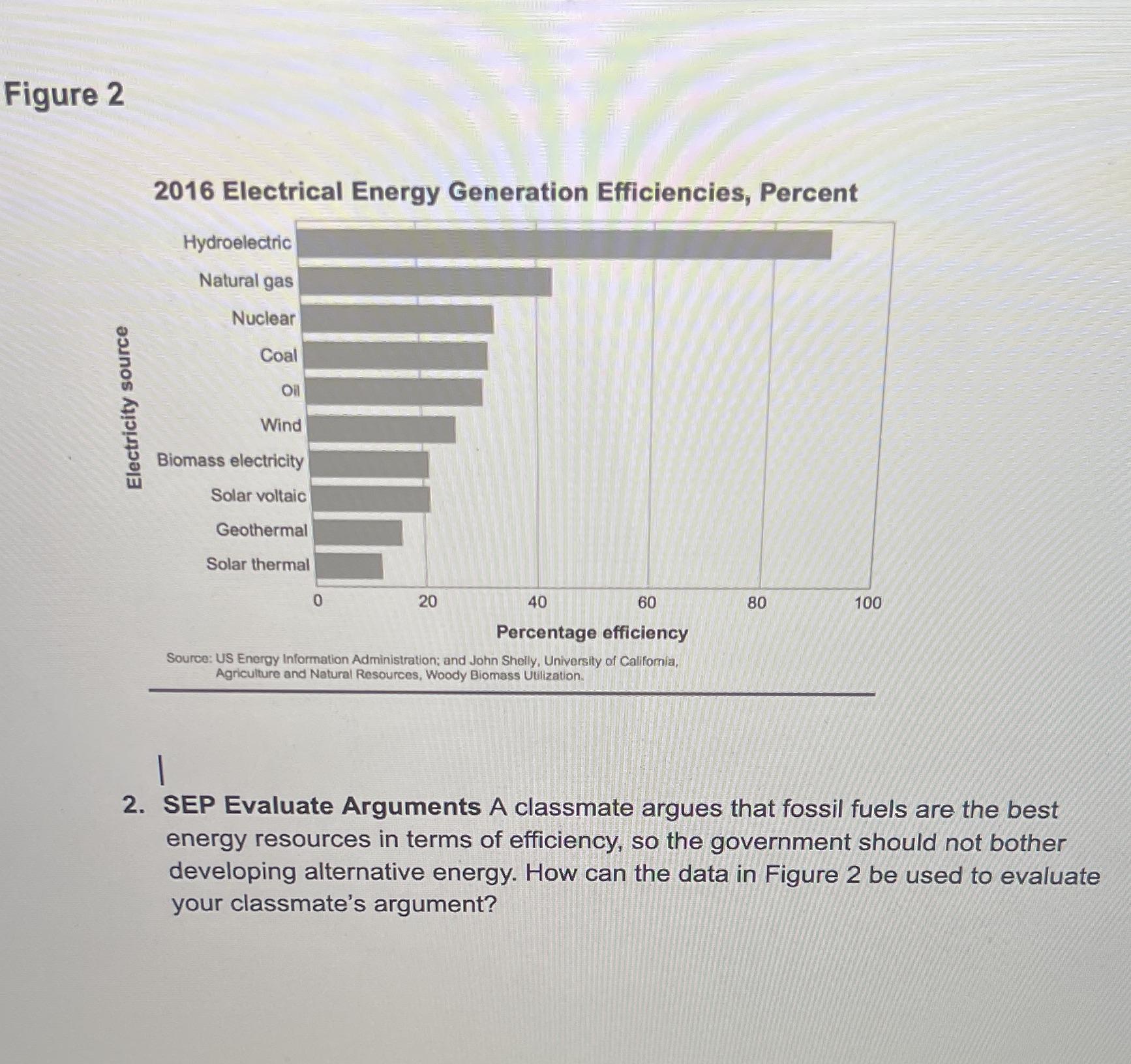
A classmate argues that fossil fuels are the best energy resources in terms of efficiency, so the government should not bother developing alternative energy. How can the data in Figure 2 be used to evaluate your classmate's argument?
Answers
Based on the figure 2, the most efficient energy source is hydroelectric, thus, Fossil fuels are not efficient energy sources and the argument is wrong.
What are fossil fuels?Fossils fuels are fuels which are obtained from fossils.
Fossil fuels include the following:
Petroleumnatural gascoalThe burning of fossil fuels such as petroleum products and coal release large amount of greenhouse gases such as carbon dioxide into the atmosphere.
This greenhouse gases are being implicated in global warming.
From figure 2, the most efficient energy source is hydroelectric.
Therefore, the argument by my classmate that fossil fuels are the best energy resources in terms of efficiency, so the government should not bother developing alternative energy is wrong.
In conclusion, fossil fuels are not efficient energy sources and should be replaced by alternative clean energy sources,
Learn more about fossil fuels at: https://brainly.com/question/10172005
#SPJ1
The formation constant for the reaction:
Ni2+(aq) + 6NH3(aq) Ni(NH3)62+(aq)
is Kf = 5.6x108 at 25 °C. Determine ??G when [Ni(NH3)62+] = 0.010M, [Ni2+] = 0.0010M, and [NH3] = 0.0050M. In which direction will the reaction proceed to achieve equilibrium?
Answers
To determine the Gibbs free energy change (∆G) for the reaction, we can use the equation:
∆G = -RT ln(K)
Where:
- R is the gas constant (8.314 J/mol·K)
- T is the temperature in Kelvin
- K is the equilibrium constant (formation constant in this case)
First, we need to convert the temperature to Kelvin. Assuming 25 °C, we have:
T = 25 + 273.15 = 298.15 K
Next, we substitute the values into the equation:
∆G = - (8.314 J/mol·K) * 298.15 K * ln(5.6x10^8)
Calculating the natural logarithm:
∆G = - (8.314 J/mol·K) * 298.15 K * 19.934
∆G = - 49390 J/mol
Since ∆G is negative, the reaction is spontaneous in the forward direction. In other words, the reaction will proceed from left to right to achieve equilibrium.
To know more about direction of reaction, visit:
brainly.com/question/12519316
#SPJ11
Explain one way that water can impact the weather and how that can affect humans.
Answers
One way that water can impact the weather is through the process of evaporation. When the sun heats up water bodies such as oceans, lakes, and rivers, water molecules become more energetic, and some of them break their bonds and rise up into the air as water vapor. This process is known as evaporation.
As water vapor rises, it cools down, and some of it condenses into tiny water droplets or ice crystals, forming clouds. These clouds can then produce precipitation, such as rain, snow, sleet, or hail, depending on the temperature and atmospheric conditions. This precipitation can be beneficial to humans as it provides water for drinking, irrigation, and other uses.
However, extreme precipitation events, such as heavy rain or snowstorms, can also lead to flooding, landslides, and other hazards, which can affect human lives and properties.
Moreover, changes in the amount and distribution of precipitation due to climate change can impact agricultural production, water availability, and the occurrence of natural disasters, such as droughts, wildfires, and hurricanes.
Therefore, understanding the role of water in the weather is essential for predicting and mitigating the impacts of extreme weather events on human societies and ecosystems.
For more question on weather click on
https://brainly.com/question/14732894
#SPJ11
what is the general principle of solubility?
Answers
Answer:
The short general principle of solubility states that "like dissolves like." Solvents that have similar polarity or charge to the solute tend to dissolve it more readily.
Solubility is the ability of a substance to dissolve based on chemical nature, intermolecular forces, and "like dissolves like" principle. Factors like particle size, temperature, and pressure affect solubility. It is expressed as the maximum amount of solute that can dissolve in a solvent.
Which one of the following processes produces a decrease in the entropy of the system?
A) freezing Fe(l) into Fe(s)
B) evaporation of liquid ethanol into gaseous ethanol
C) dissolution of LiOH(s) in water
D) melting ice to form water
E) mixing of two gases into one container
I know the answer is A but I need a detailed answer as to why that is the answer
Answers
The answer is A produces a decrease in the entropy of the system
A) we know that entropy is the measure of randomness. if randomness increases entropy also increases.
entropy order of substances : solid < liquid < gas
here conversion of liquid to solid means randomness decreases . so that entropy decreases
B) \(liquid \rightarrow gas\: conversion\) . so entropy is increases
C) dissolution of LiOH. that means LiOH molecules distributed more randomly in the solution so more disorder and more entropy
D) ice melting means : solid to liquid so entropy also increases
E) gas mixing means more disturbed in the gases more disturb will leads to more randomness and more entropy
Learn more about entropy.
brainly.com/question/13146879
#SPJ4
Which of the following molecules would have the highest boiling point?
a) hexane
b) octane
c) 2-propylpentane
d) 2-methylhexane
Answers
The molecule which would have the highest boiling point is 2-methylhexane. Thus, the correct option will be D.
What is boiling point?The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The boiling point of a liquid is a measure of its vapor pressure. The higher the boiling point, the higher the vapor pressure of the liquid, and the more heat is required to vaporize it.
The boiling point of a substance is affected by the strength and types of intermolecular forces. The stronger the intermolecular forces, the higher the boiling point. 2-methylhexane has highest boiling point because it has the highest number of carbons and branches, which contribute to its strong intermolecular forces that lead to a higher boiling point.
Therefore, the correct option is D.
Learn more about Boiling point here:
https://brainly.com/question/25777663
#SPJ11
Which compounds are bases in aqueous solution according to brønsted–lowry theory?.
Answers
According to the Brønsted-Lowry theory, a base is a substance that accepts a proton (H+ ion) from another substance in a chemical reaction.
In aqueous solution, some examples of bases include hydroxide ions (OH-), ammonia (NH3), and bicarbonate ions (HCO3-). These compounds all have lone pairs of electrons that can accept a proton, thereby forming a new bond and becoming a conjugate acid. It is important to note that the strength of a base depends on its ability to accept protons, so some bases may be weaker or stronger than others. Overall, there are many compounds that can act as bases in aqueous solution, and their behavior can be understood using the Brønsted-Lowry theory.
To know more about compound visit:
https://brainly.com/question/13516179
#SPJ11
When salt is mixed in water, what is the salt and water? A)salt is the solution, water is the solute. B)salt is the solvent, water is the solute. C)salt is the solute, water is the solvent. D)salt is the solvent, water the is the solution
Answers
Answer:
in a solution of salt in water, the solute is salt, and solvent is water.
Explanation:
C) salt is the solute, water is the solvent.
Answer:
C
Explanation:
:)
c What mass of gas is present in
48 cm of oxygen. O2
Answers
Answer:
carbon dioxide
Explanation:
because we breath in oxygen and breath out Carbon
If a p-wave arrives at 2:10:00 and the s-wave arrives at 2:15:20, how many kilometers from the epicenter is a location?
Answers
Answer:
2.67 x 10⁷km
Explanation:
Given parameters:
Time of arrival of p-waves = 2: 10: 00
Time of arrival of s-waves = 2: 15: 20
Unknown:
Distance of wave from epicenter = ?
Solution:
To find the distance of the wave from the epicenter;
Distance = 3 x 10⁸ x time interval
Time interval = Time of arrival of p-waves - Time of arrival of s-waves
= 2: 10: 00 - 2: 15: 20
= 5min 20s
= 320s
Now, we need to convert the time to hrs;
3600s = 1hr
320s = \(\frac{320}{3600}\) = \(\frac{4}{45}\)hr
So,
Distance = 3 x 10⁸ x \(\frac{4}{45}\) = 2.67 x 10⁷km
consider the following buffer solution: hcn h2o nacn h3o if koh is added, which species will react with the hydroxide ions from the koh to keep the ph from changing?
Answers
This maintains the buffer capacity of the solution and helps to resist changes in pH when KOH is added
In the given buffer solution, HCN and CN- act as a conjugate acid-base pair. When KOH is added, the hydroxide ions (OH-) react with H+ ions from HCN to form water (H2O) and produce more CN- ions. This reaction keeps the pH from changing as HCN is a weak acid and CN- is a weak base. Therefore, the species that react with the hydroxide ions to keep the pH from changing are H+ ions from HCN.
In this case, the hydroxide ions (OH-) will react with HCN, which is the acidic species in the buffer, to form water (H2O) and cyanide ion (CN-):
HCN + OH- → H2O + CN-
This reaction helps to neutralize the added hydroxide h ions and prevent the pH from increasing. The resulting cyanide ions (CN-) will then combine with the sodium ions (Na+) from NaCN, which is the conjugate base of the buffer, to form NaCN and complete the buffer system:
CN- + Na+ → NaCN
learn more about Buffer capacity here:
https://brainly.com/question/29458673
#SPJ11
.
In the equation
H2 + N2 --> NH3
NH3 is a
reactant
product
yields
catalyst
Answers
can sb helppppppppppppp

Answers
Explanation:
Copper does not react with HCl to give H2 gas.
Which answer best describes what is happening in the following redox reaction?
4Fe + 3O2 Right arrow. 2Fe2O3
This is combustion.
This is neutralization.
Iron is oxidized to form rust.
Oxygen is oxidized to form rust.
Answers
Iron is oxidized to form rust. Hence, option C is correct.
What is a redox reaction?A chemical reaction that takes place between an oxidizing substance and a reducing substance.
Chemical reaction: Fe + O₂ → Fe₂O₃.
Oxidation half reaction:
Fe⁰ → Fe⁺³ + 3e⁻
4Fe⁰ → 4Fe⁺³ + 12e⁻
Reduction half-reaction:
O₂⁰+ 4e⁻ → 2O⁻²
3O₂⁰+ 12e⁻ → 6O⁻²
Balanced chemical reaction:
4Fe + 3O₂ → 2Fe₂O₃.
Oxidation is an increase of oxidation number, iron is oxidized from oxidation number 0 (Fe) to oxidation number +3 (in rust Fe₂O₃).
Learn more about redox reaction here:
https://brainly.com/question/13293425
#SPJ1
Answer:Iron is oxidized to form rust.
Explanation:
which of the following is not a factor that changes the reaction quotient of a chemical system at equilibrium? select the correct answer below: a decrease in the concentration of a product an increase in volume the introduction of a catalyst an increase in the concentration of a product
Answers
The addition of a catalyst from the list below will not alter the reaction rate of an equilibrium chemical system.
Which of the following variables does not effect changes in chemical equilibrium?The chemical equilibrium is unaffected by a catalyst. That just quickens a response. In actuality, a catalyst quickens both the forward and backward reaction. As we increase the pressure, the response changes in a way to offset that effect, so changing the pressure has no influence on the equilibrium constant.
What variables affect the chemical reaction's equilibrium?The equilibrium position of a reversible reaction can be impacted by variations in concentration, temperature, and pressure. Chemical reactions are equilibrium reactions.
To know more about reaction rate visit:-
https://brainly.com/question/30546888
#SPJ1
What is the Actual vs. Ideal State of Old Spice
External Search and Internal Search of Old Spice
Answers
Assessing the actual vs. ideal state of Old Spice's external search and internal search requires considering factors such as the brand's marketing efforts, information availability, online presence, customer reviews, and the overall consumer perception and loyalty towards the brand.
To evaluate the actual vs. ideal state of Old Spice's external search and internal search, we need to understand these concepts in the context of the brand and its marketing strategies.
External Search:
External search refers to the process by which consumers gather information from external sources to aid in their decision-making. In the case of Old Spice, the external search would involve consumers seeking information about Old Spice products, such as their features, benefits, pricing, availability, and customer reviews.
Actual State: The actual state of Old Spice's external search would depend on the effectiveness of its marketing efforts in providing readily available and easily accessible information to consumers. This could include advertising campaigns, product displays in stores, online presence, social media engagement, and customer reviews. The actual state would also be influenced by the brand's visibility and reputation among consumers.
Ideal State: The ideal state of Old Spice's external search would involve a comprehensive and user-friendly information ecosystem. This could include an informative and updated website, interactive online platforms, engaging social media presence, informative product descriptions, and positive customer reviews. The ideal state would aim to provide consumers with easy access to accurate and relevant information about Old Spice products, ultimately facilitating their decision-making process.
Internal Search:
Internal search refers to the process by which consumers draw upon their own knowledge and past experiences to evaluate and make decisions about a product or brand. In the context of Old Spice, internal search would involve consumers relying on their prior familiarity with the brand, its reputation, personal experiences with Old Spice products, and any pre-existing preferences or biases they may have.
Actual State: The actual state of Old Spice's internal search would depend on the brand's ability to create positive associations and experiences among consumers. It would also depend on the brand's efforts to maintain a consistent and recognizable image that consumers can recall and rely on during their decision-making process.
Ideal State: The ideal state of Old Spice's internal search would involve consumers having strong positive associations, experiences, and brand loyalty. This would mean that consumers readily recall their positive experiences with Old Spice products, have a favorable perception of the brand, and consider Old Spice as a preferred choice when evaluating similar products in the market.
To know moe about marketing strategies
https://brainly.com/question/31854392
#SPJ11
a sample of f-18 has an initial decay rate of 1.5 * 105>s. how long will it take for the decay rate to fall to 2.5 * 103>s? (f-18 has a half-life of 1.83 hours.)
Answers
The time taken for the decay rate to fall to 2.5×10³, given that the sample has a half-life of 1.83 hours is
How do i determine the time taken?We'll begin by obtaining the number of half lives that has passed during the decay. Details below
Initial decayrate (A₀) = 1.5×10⁵ Final decay rate (A) = 2.5×10³Number of half-lives (n) =?2ⁿ = A₀ / A
2ⁿ = 1.5×10⁵ / 2.5×10³
2ⁿ = 60
Take the log of both sides
Log 2ⁿ = log 60
nLog2 = log 60
Divide both sides by log 2
n = log 60 / log 2
n = 5.907
Finally, we shall determine the time taken. Details below
Half-life of f-18 (t½) = 1.83 hoursNumber of half-lives (n) = 5.907 Time taken (t) =?n = t / t½
5.907 = t / 1.83
Cross multiply
t = 5.907 × 1.83
t = 10.81 hours
Thus, the time taken is 10.81 hours
Learn more about time to decay:
https://brainly.com/question/20629414
#SPJ1
Of the choices below, which energy resource has the highest net energy ratio for transportation?
A. Ethanol from sugar cane residue
B. Natural gas
C. Oil shale
D. Gasoline
E. Coal liquefaction
Answers
Among the choices provided, natural gas (option B) typically has the highest net energy ratio for transportation. The correct option is b).
The net energy ratio refers to the ratio of the energy obtained from a resource to the energy invested in its production, including extraction, processing, and transportation. A higher net energy ratio indicates a more efficient and sustainable energy source.
Natural gas is known for its relatively high net energy ratio compared to other fossil fuels. It is primarily composed of methane and is often extracted from underground reservoirs. Natural gas has a lower carbon content and produces fewer greenhouse gas emissions compared to coal or oil. Its combustion also releases fewer pollutants, making it a cleaner-burning fuel.
Ethanol from sugar cane residue (option A) is a renewable energy source that can be used for transportation. However, the net energy ratio of ethanol can vary depending on the production process, feedstock, and energy inputs required for conversion. While ethanol has some advantages, such as reduced greenhouse gas emissions, its net energy ratio can be lower due to the energy-intensive processes involved in cultivation, processing, and transportation.
Oil shale (option C) and coal liquefaction (option E) involve extracting hydrocarbons from unconventional sources. These processes are typically energy-intensive and have lower net energy ratios compared to conventional oil or natural gas extraction.
Gasoline (option D) is derived from crude oil and has a lower net energy ratio compared to natural gas. Crude oil extraction, refining, and distribution processes require significant energy inputs.
In summary, among the options provided, natural gas generally has the highest net energy ratio for transportation due to its relatively efficient extraction, lower carbon content, and cleaner combustion compared to other fossil fuels. Option b) is the answer.
Know more about natural gas here:
https://brainly.com/question/12200462
#SPJ11
Sample A is measured to have a mass of 7.3g and sample B has a mass of 8.28g. Which measurement best describes their combined mass?
A.16g
B.15.58g
c.15.5g
D15.6g
Answers
Calculate the number of milliliters of 0.444 M required to precipitate all of the ions in 107 mL of 0.699 M solution as . The equation for the reaction is:
Answers
333.3 milliliters of 0.444 M KOH required to precipitate all of the Pb⁺² ions in 107 mL of 0.699 M Pb(NO₃)₂ solution as Pb(OH)₂.
What is Stoichiometry ?Stoichiometry helps us use the balanced chemical equation to measure quantitative relationships and it is to calculate the amounts of products and reactants that are given in a reaction.
What is Molarity ?Molarity (M) is defined as the number of moles of solute dissolved in 1L of solution. Molarity is also known as Molar Concentration. The S.I unit of Molarity is molar (M) or mol/L.
Molarity = \(\frac{\text{Number of moles}}{\text{Volume of solution (in L)}}\)
Now put the values in above formula to find the number of moles of Pb(NO₃)₂
Molarity = \(\frac{\text{Moles of}\ Pb(NO_{3})_2}{\text{Volume of solution (in L)}}\)
Moles of Pb(NO₃)₂ = Molarity × Volume
= 0.699M × 0.107 L [1ml = 0.001 L]
= 0.074 moles
The given balanced chemical equation is
Pb(NO₃)₂(aq) + 2 KOH(aq) → Pb(OH)₂(s) + 2 KNO₃(aq)
The mole ratio of KOH to Pb(NO₃)₂ is 2:1.
The mole of KOH = 0.074 mol × 2
= 0.148 moles
Now put the value in above formula to find the volume of KOH
Molarity = \(\frac{\text{Number of moles}}{\text{Volume of solution (in L)}}\)
Volume of KOH = \(\frac{\text{Number of mole of KOH}}{\text{Molarity}}\)
Volume of KOH = \(\frac{0.148\ mol}{0.444\ M}\)
= 0.333 L
= 333.3 mL [1L = 1000 mL]
Thus from the above conclusion we can say that 333.3 milliliters of 0.444 M KOH required to precipitate all of the Pb⁺² ions in 107 mL of 0.699 M Pb(NO₃)₂ solution as Pb(OH)₂.
Learn more about the Molarity here: https://brainly.com/question/26873446
#SPJ4
Disclaimer: The question was given incomplete on the portal. Here is the complete question.
Question: Calculate the number of milliliters of 0.444 M KOH required to precipitate all of the Pb⁺² ions in 107 mL of 0.699 M Pb(NO₃)₂ solution as Pb(OH)₂. The equation for the reaction is:
Pb(NO₃)₂(aq) + 2 KOH(aq) → Pb(OH)₂(s) + 2 KNO₃(aq)
_________ mL KOH.
hcl(g) and nh3(g) react to form nh4cl, a white solid. if nh3(g) and hcl(g) are introduced at opposite ends of a 60.0cm tube, how far from the hcl end will the white ring of nh4cl form?
Answers
The white ring of the NH₄Cl is formed at 24.3cm far from Hcl end.
What is grahams law of diffusion?
According to Graham's law, a gas's rate of diffusion or effusion is inversely related to the square root of its molar mass.
What is solid?
Solid ground is a substance that is hard, firm, or compact. having relative firmness, particle coherence, or form permanence as matter that is not liquid or gaseous: solid particles suspended in a liquid. regarding such a matter: Ice is water when it's solid.
According to grahams law of diffusion, rate of diffusion is inversely propotional to square root of its density and molar mass.
r ∝ 1/ \(\sqrt{d}\) and γ ∝ 1/ \(\sqrt{M}\)
γ NH₃/ γ HCl = \(\sqrt{M HCl / M NH3}\) = \(\sqrt{36.5/17}\)
1.465/ 1
given that
tube length = 60.0cm
We known that rate = distance / time
Time = distance / rate
let X be the distance travelled by HCl and
60-X be the distance travelled by NH₃
Time taken for HCl = Distance travelled by HCl/ rate of diffusion of Hcl
Time taken for NH₃ = Distance travelled by NH₃ / rate of diffusion of Hcl
Since both gases are introduced at the same time and travelled same time
distance travelled by HCl (dHCl) / rate of diffusion of HCl = distance travelled by NH₃ ( dNH₃) / rate of diffusion of NH₃ (r NH₃ )
γ NH₃ / γ HCl = d NH₃ / d HCl = 60-X /X = 1.465/1
60-X = 1.465X
60=2.465X
X=60/ 2.465= 24.34 cm
Therefore, white ring of the NH₄Cl is formed at 24.3cm far from Hcl end.
Learn more about grahams law of diffusion from the given link.
https://brainly.com/question/22359712
#SPJ4
The disappearing spoon chapter one summary
Answers
Chapter One of the book "The Disappearing Spoon" by Sam Kean is titled "Ruthenium, Rhodium, and Palladium." This chapter explores the fascinating history, properties, and uses of these three elements from the periodic table.
The chapter begins with the story of a chemical spill in the town of Norilsk, Russia, which is home to the world's largest nickel mine. The spill caused massive environmental damage and raised concerns about the toxic effects of metals.
This incident sets the stage for the exploration of elements that possess unique properties and have played significant roles in scientific and industrial advancements.
The author then introduces the readers to the periodic table and its significance in understanding the behavior and characteristics of elements. He explains the arrangement of elements and how they are grouped based on their chemical properties.
Moving on, Kean delves into the history and properties of ruthenium, rhodium, and palladium. He shares interesting anecdotes about their discoveries, including the challenges faced by scientists in isolating and identifying these elements. The author highlights the rarity and value of these metals and their importance in various fields, such as catalysis, electronics, and jewelry making.
Furthermore, Kean discusses the cultural and societal impact of these elements, including their use in the automotive industry, where palladium plays a crucial role in catalytic converters. He also explores the darker side of these elements, such as their involvement in illegal activities, including theft and smuggling.
Overall, Chapter One of "The Disappearing Spoon" provides an engaging introduction to the world of elements and sets the stage for further exploration of the periodic table and its fascinating stories.
For more such question on Palladium visit;
https://brainly.com/question/28603477
#SPJ8