draw the neutral organic product for the reaction shown. show stereochemistry clearly. the starting material is an alkyne where one carbon is bonded to ethyl and the other alkyne carbon is bonded to tert butyl. the starting material is converted to the product in 2 steps. the first reagent is 2 equivalents of lithium in ethylamine at minus 78 degrees celsius. the second reagent is n h 4 c l.
Answers
The other alkyne carbon is connected to tert-butyl, and one carbon is attached to ethyl. Alkynes are unsaturated hydrocarbons in which the two carbon atoms are connected by a triple bond.
Alkynes Addition Reactions. A terminal carbon-carbon triple bond is one that is found at the end of a ethyl chain, although it can also be found at any unbranched position along a carbon chain. The Alkynes are saturated hydrocarbons, which are hydrocarbons with only one ethyl bond. One or more double bonds exist between the carbon atoms in alkenes. One or more triple bonds exist between the carbon atoms in alkynes. The electron systems in the rings of aromatic hydrocarbons are delocalized.
learn more about alkynes here:
https://brainly.com/question/24270289
#SPJ4

Related Questions
ultraviolet and ____ rays are harmful rays emitted during arc welding.
Answers
Ultraviolet and infrared rays are harmful rays emitted during arc welding.
During arc welding, an intense electric arc is generated, producing various types of radiation. Ultraviolet (UV) rays are one form of harmful radiation emitted during the welding process. UV rays have a shorter wavelength than visible light and can cause damage to the skin and eyes, leading to sunburn, skin aging, and even long-term health risks such as skin cancer.
In addition to UV rays, infrared (IR) rays are also emitted during arc welding. IR rays have longer wavelengths than visible light and can generate heat. Prolonged exposure to IR rays can cause thermal burns and skin damage.
To protect against the harmful effects of UV and IR radiation during arc welding, welders typically use personal protective equipment (PPE) such as welding helmets with shaded lenses or filters. These lenses are designed to block or reduce the transmission of harmful UV and IR rays while allowing safe levels of visible light to pass through, ensuring the welder's safety.
Learn more about Ultraviolet and infrared rays from the link given below.
https://brainly.com/question/3628208
#SPJ4
What type of molecule is pentanal?
o a. ester
b. alcohol
o c. ketone
o d. aldehyde
i will mark brainliest!!!!
Answers
Name some of the important rocks found in Nepal
Answers
Answer:
shale
sandstone
limestone
Explanation:
These fossiliferous rocks are extensively developed in Nepal, particularly in Thak Khola (Mustang), Manang, and Dolpa. This zone is around 40 kilometers broad and is made mostly of fossiliferous sedimentary rocks.
Explain the significance of polar and non-polar amino acids
Answers
The significance of polar and non-polar amino acids lies in their interactions within a protein structure. Polar amino acids are typically found on the surface of the protein, where they interact with water molecules and other polar molecules. Non-polar amino acids, on the other hand, are typically found in the interior of the protein, where they interact with other non-polar amino acids through hydrophobic interactions.
Amino acids are the building blocks of proteins, and they can be categorized as either polar or non-polar. Polar amino acids have a hydrophilic (water-loving) nature due to their polarity, while non-polar amino acids have a hydrophobic (water-fearing) nature due to their lack of polarity.
The balance between polar and non-polar amino acids is crucial in determining the overall structure and function of a protein. If there are too many polar amino acids in the interior of a protein, it may become unstable and unfold. Conversely, if there are too many non-polar amino acids on the surface of a protein, it may not be able to interact effectively with other molecules.
Overall, the significance of polar and non-polar amino acids lies in their ability to contribute to the stability and function of proteins. Understanding the properties of these amino acids is important in fields such as biochemistry and drug development.
To know more about the polar and non-polar amino acids refer here :
https://brainly.com/question/31234933#
#SPJ11
imagine a reaction that can replace one hydrogen atom of an alkane at random with a chlorine atom
Answers
By replacing one hydrogen atom of 2,2-dimethylbutane with a chlorine atom, 3 different compounds can be obtained, ignoring optical isomers.
To determine the number of different compounds that can be formed by replacing one hydrogen atom of 2,2-dimethylbutane with a chlorine atom, we need to identify the unique hydrogen positions in the molecule.
2,2-dimethylbutane has the following structure: CH3-C(CH3)2-CH2-CH3
There are three unique hydrogen positions:
1. Hydrogen atoms on the two terminal CH3 groups (methyl groups) - There are 6 hydrogen atoms in total at this position (3 on each methyl group), but they are equivalent. Replacing one of them will create the same compound.
2. Hydrogen atoms on the central C(CH3)2 carbon - There are 2 equivalent hydrogen atoms in this position.
3. Hydrogen atoms on the CH2 group - There are 2 equivalent hydrogen atoms in this position.
Now let's consider the possible compounds that can be formed:
1. Replace one hydrogen atom from the terminal methyl groups: CH2Cl-C(CH3)2-CH2-CH3
2. Replace one hydrogen atom from the central C(CH3)2 carbon: CH3-C(CH3)2-CHCl-CH3
3. Replace one hydrogen atom from the CH2 group: CH3-C(CH3)2-CH2-CH2Cl
To know more about hydrogen
https://brainly.com/question/28937951
#SPJ11
What is the mass percentage of C in codeine, C₁₈H₂₁NO₃? Provide an answer to two decimal places
Answers
The mass percentage of C in codeine, C₁₈H₂₁NO₃, is 63.16%.
To calculate the mass percentage of C in codeine, we need to find the molar mass of the compound first.
Molar mass of C₁₈H₂₁NO₃ = (18 x 12.011) + (21 x 1.008) + (1 x 14.007) + (3 x 15.999) = 299.37 g/mol
Next, we need to find the mass of the carbon atoms in one mole of codeine. Since there are 18 carbon atoms in one mole of codeine, we can multiply the molar mass by the number of carbon atoms and divide by the total molar mass of the compound:
Mass of carbon atoms = 18 x 12.011 g/mol = 216.198 g/mol
Mass percentage of C = (mass of carbon atoms / molar mass of codeine) x 100% = (216.198 g/mol / 299.37 g/mol) x 100% = 63.16%
As a result, codeine has a mass proportion of C of 63.16%.
To know more about the Codeine, here
https://brainly.com/question/14343238
#SPJ4
How does ionic bonding takes place?
A.Two non-metallic elements of different kinds form strong forces of attraction.
B.Two non- metallic elements of the same kind of form strong forces of attraction.
C. A non-metallic element like fluorine is attracted to a metallic element like sodium.
D. A metallic element like sodium transfer an electron to a non-metallic element like fluorine
Answers
Answer:
Explanation:
Option D is the correct answer
How many moles are there in 2.3 x 1024 atoms of
sulfur?
Answers
Explanation:
The steps given in the question are incorrect.
Step 1 should be convert atoms to moles (n). Step 2 should be convert moles (n) to mass (m).
Step 1
Use dimensional analysis to convert the number of atoms to moles.
1 mole atoms = 6.022 × 10²³ atoms
n(Ag) = 2.3 × 10²⁴ Ag atoms × (1 mol Ag/6.022 × 10²³ Ag atoms) = 3.8193 mol Ag
Step 2
Convert the moles of Ag to mass.
mass (m) = moles (n) × molar mass (M)
n(Ag) = 3.8193 mol Ag
M(Ag) = atomic weight on the periodic table in g/mol = 107.868 g Ag/mol Ag
m(Ag) = 3.8193 mol × 107.868 g/mol = 412 g Ag = 410 g Ag rounded to two significant figures
The mass of 2.3 × 10²⁴ Ag atoms is approximately 410 g.
Answer:
\(\boxed {\boxed {\sf 3.8 \ moles \ of \ sulfur}}\)
Explanation:
We are asked to convert a number of atoms to moles.
We can convert atoms to moles using Avogadro's Number, which is 6.022 × 10²³. This is the number of particles (atoms, molecules, formula units, etc.) in 1 mole of a substance. In this problem, the particles are atoms of sulfur. There are 6.022 ×10²³ atoms of sulfur in 1 mole of sulfur.
We use dimensional analysis to convert atoms to moles. This involves setting up ratios. Use Avogadro's Number and the underlined information to make a ratio.
\(\frac {6.022 \times 10^{23} \ atoms \ S}{1 \ mol \ S}\)
We are converting 2.3 ×10²⁴ atoms of sulfur to moles, so we multiply by this value.
\(2.3 \times 10^{24} \ atoms \ S *\frac {6.022 \times 10^{23} \ atoms \ S}{1 \ mol \ S}\)
Flip the ratio. It is equivalent, but it allows the units of atoms of sulfur to cancel.
\(2.3 \times 10^{24} \ atoms \ S *\frac {1 \ mol \ S}{6.022 \times 10^{23} \ atoms \ S}\)
\(2.3 \times 10^{24} *\frac {1 \ mol \ S}{6.022 \times 10^{23} }\)
\(\frac {2.3 \times 10^{24} }{6.022 \times 10^{23} } \ mol \ S\)
\(3.819329127 \ mol \ S\)
The original measurement of atoms (2.3 ×10²⁴) has 2 significant figures, so our answer must have the same. For the number we calculated that is the tenths place. The 1 in the hundredths place to the right (3.819329127) tells us to leave the 8 in the tenths place (3.819329127).
\(3.8 \ mol \ S\)
2.3 ×10²⁴ atoms of sulfur is equal to approximately 3.8 moles of sulfur.
I’m unsure how to answer this with sig figs in mind:Use scientific notation to to express this quantity: 131. mg

Answers
Use scientific notation to to express this quantity: 131. mg
Explanation:
131. mg has 3 SF
131. mg = 1.31 * 100 mg
131. mg = 1.31 * 10^2 mg
Answer: 1.31 * 10^2 mg

Combustion and corrosion are similar reactions because they both... A) release Carbon Dioxide as a product B) release oxygen as a product C) require oxygen as a reactant D) Have a metal as a reactant
Answers
Answer:
B. release oxygen as a product
Explanation:
Sketchpad
a chemist dilutes 2.0 l of a 1.5 m solution with water until the final volume is 6.0 l. what is
the new molarity of the solution?
show your work
Answers
The new molarity of the solution after dilution is 0.5 M.
To solve this problem, we can use the formula:
\(M_2 = M_1V_1 / V_2\)
where \(M_1\) and \(V_1\) are the initial molarity and volume of the solution, and \(M_2\) and \(V_2\) are the final molarity and volume of the diluted solution.
In this case, we have:
\(M_1\) = 1.5 M
\(V_1\) = 2.0 L
\(V_2\) = 6.0 L
We want to find the final molarity, \(M_2\).
Using the formula, we can solve for \(M_2\):
\(M_2 = M_1V_1 / V_2\)
Substituting the given values, we get:
\(M_2\) = (1.5 M) × (2.0 L) / (6.0 L) = 0.5 M
Therefore, the new molarity of the solution is 0.5 M.
To know more about initial molarity, here
brainly.com/question/18084320
#SPJ1
In table below, there are descriptions of an experiment on samples of three different chemical elements. Decide whether the element is a metal or nonmetal, if you can. If there is not enough information to decide, choose can't decide in the third column element description metal or nonmetal? 5 ? Element 1 is a shiny silvery-gray solid. Wires are fastened to each side of a 2 cm slab of it, and an ordinary household 9 V battery is hooked up so that it can feed electricity through the slab to an LED. The LED glows brightly. O metal nonmetal (can't decide) Element 2 is a hard silvery-gray solid. A 10. g cube of it is tapped lightly with a small hammer. One corner of the cube breaks off into 3.4 pieces and a collection of small bits. metal nonmetal (can't decide)
Answers
element 1 is non metals, Hard Solid cube
Breaks into pieces of small bits when tapped with hammers- Brittle nature
element 2 is metals ,Moderately soft solid (may be soft metal).5*5 cm square with only 1mm thickness (form of sheets) : malleable nature.
On heating, it got softened : good conductor of heat.
METALS:
Metals occurs in solid state except mercury which is liquid form, rest all the metals are solid form. Metals are shiny, hard and strong opaque materials except sodium and potassium. Metals can be cut by knife. Metals are ductile in nature i.e., they can be stretched into wires. They are malleable in nature which can be cut into sheets. They have high density, high boiling and melting points. They are good conductors of electricity and heat.
NON-METALS:
Non-metals are soft/ hard and brittle in nature i.e., they can be break into pieces.
They are not ductile and malleable.
They have low density, boiling and melting points.
They are poor conductors of electricity and heat.
Learn more about metals here:
https://brainly.com/question/28650063
#SPJ4
In the reaction shown below, ____________ acts as a base because ___________. HCO3- + H2O à H3O+ + CO32-
Answers
Answer: In the reaction \(HCO^{-}_{3} + H_{2}O \rightarrow H_{3}O^{+} + CO^{2-}_{3}\), \(H_{2}O\) acts as a base because it has accepted a hydrogen ion or proton.
Explanation:
According to Arrhenius, species which dissociate to give hydrogen ions when dissolved in a solvent like water are called acid.
A species which readily accepts a hydrogen ion or proton is called a base.
For example, \(HCO^{-}_{3} + H_{2}O \rightarrow H_{3}O^{+} + CO^{2-}_{3}\)
Here, \(HCO^{-}_{3}\) is donating hydrogen ion. So, it is an acid whereas \(H_{2}O\) is accepting the hydrogen ion. Hence, \(H_{2}O\) is a base.
Thus, we can conclude that in the reaction \(HCO^{-}_{3} + H_{2}O \rightarrow H_{3}O^{+} + CO^{2-}_{3}\), \(H_{2}O\) acts as a base because it has accepted a hydrogen ion or proton.
what kind of oxide is formed when a piece of sodium is dropped in the water
Answers
Answer:
Sodium oxide is the product
Explanation:
4Na+O2->2Na2O
Summary
1.
Water is the most abundant substance on the surface of the earth.
About 71% of the earth's surface is covered by water.
2. Hydrogen is prepared in the laboratory by the action of zinc on
dilute hydrochloric acid or dilute sulphuric (VI) acid.
3. Hydrogen burns in oxygen to produce water. Water is an oxide
of hydrogen.
4. Active metals react with cold water to produce hydrogen gas
and the hydroxide of the metal in solution. Less active metals
react with steam to produce hydrogen and the oxide of the metal.
Copper and lead do not react with water.
5. Hydrogen is a reducing agent. It removes combined oxygen
from metal oxides of the less reactive metals.
6.
Reduction is the loss of oxygen from a compound. Oxidation is
the gain of oxygen by a substance.
7.
A reducing agent is a substance which removes oxygen from
another substance. An oxidising agent is a substance which gives
out oxygen to another substance.
when
water changes the
colour of coppe - 11
Revision Exercise sulphatorom blueto
1. (a) State the chemical tests for presence of water.
water change the color
(b) State the test, which is used to show that water is pure. Chistide paper from b
of copper anhydrous cobele
2.
Describe an experiment to show that water is an oxide of hydrogen.t non
3. State the precautions that must be taken when carrying out experiments with hydrogen.
Why is it not advisable to use iron in making steam boilers?
Write a word equation for a reaction in which hydrogen acts as a reducing agent.
Name the products formed when kerosene is burned in air.
State what is observed when a small piece of potassium is placed in water. Write a
word equation for the reaction.
Draw a labelled diagram to show how a reaction between steam and magnesium should
be carried out.
Describe how dry hydrogen is prepared in the laboratory.
Property of the Government of Kenya
Potassium
sodium
Calcim
Magne
Alun
Corbi
Zin
Answers
Explanation:
I know only one.You are in form.....

What is the∆S° of 0₂
Answers
Answer:0
Explanation: zero because it is the most stable form of oxygen in its standard state
In an appropriately designed experiment, a scientist is able to test the effect of
Answers
A) a single variable B) multiple variables
C) the hypothesis D) scientific observations
This answer is A. a single variable
Hope this helps!
Predict what would happen if a scientist added potassium to a dilute acid.
Answers
Potassium reacts with dilute hydrochloric acid to give potassium chloride and hydrogen gas. Heating small pieces of Potassium in air results in the substance melting without any flame being seen and turning instantly into a mixture of potassium peroxide and potassium super oxide.
A Potassium Reaction involves a process in which Potassium is mixed with another substance which react to form something else. Reactions are manifested by the disappearance of properties characteristic of Potassium and the appearance of new properties in the new substance or Compound.
The substances initially involved in a reaction are called reactants or reagents. The most important of the Potassium compounds is Potassium chloride (KCl) which is used in the production of fertilizers and chemicals and also as a salt substitute. Other important compounds are Potassium nitrate (KNO3), also known as saltpeter which is used in the production of gunpowder, fertilizers and pyrotechnics and Potassium hydroxide (KOH) is used to make detergents and soaps. Reactions are described with Chemical Formula and Equations.
Fill in the blank with the correct term. The part of one type of amino acid that makes it different from another type is the .______
Answers
The part of one type of amino acid that makes it different from another type is the side chain (or R group).
A side chain is a group of atoms that are attached to the alpha carbon atom of the amino acid and is composed of either a hydrogen atom, an alkyl group (a carbon atom with several hydrogen atoms attached), an aryl group (a carbon atom with several hydrogen atoms and a benzene ring attached), an acidic group (a carboxyl group or a sulfhydryl group), or a basic group (an amine group, a guanidinium group, or an imidazole group).
Each amino acid has a unique side chain, which is what gives it its unique chemical and physical properties and distinguishes it from other amino acids. The side chain is also what determines the amino acid's behavior in different environments, such as its solubility, reactivity, and charge.
learn more about amino acid:
https://brainly.com/question/28362783
#SPJ4
The part of amino acids that makes them different from one another is R- Group.
Amino acids are organic compounds. Its molecules combine to form proteins and proteins are called the building blocks of life. Every amino acid has a hydrogen atom present in them along with a carboxyl group, an alpha-amino group, and also the R-group.
R -Group makes the side chain. The side chain present in each Amino acid is the only unique feature that differentiates them. The R groups have a variety of sizes, shapes, charges, and reactivities. Thus, allowing them to be grouped according to the chemical properties of their side chains.
Therefore, the only main factor that makes every amino acid different is the presence of the R-group.
Read more about Amino Acid at:
https://brainly.com/question/28362783
WILL GIVE BRAINLIEST, Which of the following would have the greatest amount of surface area?
A. 1 cubic foot of watermelons
B. 1 cubic foot of matchbox cars
C. 1 cubic foot of loose sand
D. A 1 cubic foot block of concrete
Answers
Answer:
C. 1 cubic foot of loose sand
Explanation:
For many objects having equal volume , surface area will be maximum
of the object which has spherical shape .
But when a sphere is broken into tiny small spheres , total surface area of all the small spheres will be more than surface area of big sphere .
Hence among the given option , surface area of loose sand will have greatest surface area . Loose sand is equivalent to small spheres .
Answer:
the answer would be C 1 cubic foot of loose sand.
The Dead Sea is the saltiest sea in the world. It contains 332 gramsof salt per 1000 grams of water.What is the concentration in parts per million (ppm)?0.332 ppm332 ppm332,000 ppmo33,200 ppm
Answers
Answer
The concentration in parts per million (ppm) = 332,000 ppm.
Explanation
Given:
Mass of salt solute = 332 grams
Mass of water solvent = 1000 grams
What to find:
The concentration in parts per million (ppm).
Solution:
The concentration in parts per million (ppm) formula is:
\(ppm=\frac{mass\text{ }of\text{ }solute}{mass\text{ }of\text{ }solvent}\times10^6\)Plugging the values of the given parameters into the formula:
\(ppm=\frac{332}{1000}\times10^6=332,000ppm\)The concentration in parts per million (ppm) = 332,000 ppm.
Which term describes a mixture of 48 percent iron, 22 percent chromium, and 30 percent palladium?
Answers
Alloy.
Why do we believe that these strange quantum laws are correct? Group of answer choices Experiments show they're sometimes right. There haven't been any experiments done on them because they can't be tested, so we just think they're right. In experiment after experiment, they're always right. We actually don't believe they're right, we just like to talk about them.
Answers
Answer:
In experiment after experiment, they're always right
Explanation:
In science, nothing is accepted unless it can be supported by empirical evidence, this includes the quantum laws.
A plethora of experiments have confirmed these quantum laws to be correct and reliable.
Hence, all the quantum laws are backed up by plenty scientific evidence that show that the laws are indeed correct.
On which side would the equilibrium lie for the following reaction? (Hint: Assign the species as acids/bases first!)H_{3}*P*O_{4} +NH 3 rightleftharpoons NH 4 ^ + +H 2 PO 4 ^ -
p*K_{a}*s H_{3}*P*O_{4} = 2.2 N*H_{3} = 33 N H 4 ^ + =9.8, H_{2}*P O 4 ^ - =7.2
a.To the left because the reactants are stronger acids and bases.
b. To the left because the products are stronger acids and bases.
c. To the right because the reactants are stronger acids and bases.
d. To the right because the products are stronger acids and bases.
Answers
The equilibrium would lie to the right for the following reaction: H_{3}PO_{4} + NH_{3} \rightleftharpoons NH_{4}^{+} + H_{2}PO_{4}^{-}. This is because the reactants, H_{3}PO_{4} and NH_{3}, are stronger acids and bases than the products, NH_{4}^{+} and H_{2}PO_{4}^{-}. The pK_{a} values for the reactants are 2.2 and 33, respectively, while the pK_{a} values for the products are 9.8 and 7.2, respectively. Since the pK_{a} values for the reactants are lower, they are stronger acids and bases and the reaction will favor the formation of the weaker acids and bases, which are the products. Therefore, the correct answer is option c. To the right because the reactants are stronger acids and bases
The acid and base strength can be quantified using the acid dissociation constant (Ka) and the base dissociation constant (Kb). The Ka and Kb values for H3PO4 and NH3 are:
Ka(H3PO4) = [H+][H2PO4-]/[H3PO4]
Kb(NH3) = [NH4+][OH-]/[NH3]
Since the value of Ka for H3PO4 is very small, it indicates that H3PO4 is a weak acid. Similarly, the value of Kb for NH3 is also small, indicating that NH3 is a weak base.
Now, we need to compare the strength of the acid-base pairs on each side of the equation:
On the left-hand side, the acid-base pair is H3PO4/NH3, which is a weak acid and a weak base, respectively.
On the right-hand side, the acid-base pair is NH4+/H2PO4-, which is a weak acid and a weak base, respectively.
Since both pairs are weak acids and weak bases, it is difficult to predict the direction of the equilibrium solely based on acid-base strength.
However, we can use the values of the equilibrium constants (Ka and Kb) to determine the direction of the equilibrium. The equilibrium constant for the reaction is given by:
Keq = [NH4+][H2PO4-]/[H3PO4][NH3]
Substituting the given values, we get:
Keq = (9.8 × 7.2)/(2.2 × 33) = 0.838
Since the value of Keq is less than 1, it indicates that the equilibrium lies towards the left-hand side of the equation. Therefore, the correct answer is:
a. To the left because the reactants are stronger acids and bases.
Read more about equilibrium here:https://brainly.in/question/220726
#SPJ11
what does the strength of gravity mean
Answers
Answer:
On Earth all bodies have a weight, or downward force of gravity, proportional to their mass, which Earth's mass exerts on them.
Answer:
um lets say its weight and force
Explanation:
it consist of pulling an individual object or living being to pull in a sub planet or lets say it pulls you from a land that doesn't make u float
0-gravity= to floating experience so yeah
What are some possible factors that must remain constant during the testing
Answers
Answer:
Four basic components that affect the validity of an experiment are the control, independent and dependent variables, and constants. These basic requirements need to be present and identified to consider an experiment valid.
The element with the chemical symbol ______ is a nonmetal help me please

Answers
Answer:
O
Explanation:
O is in the red which says nonmetal
Can someone help please

Answers
Answer:
To achieve stability
Explanation:
Chemical reactions happen so that the atoms of the elements involved in the chemical reaction will become stable and therefore achieve stability.
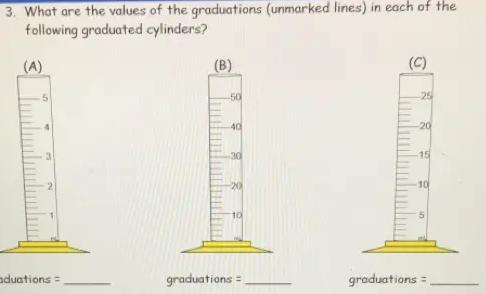
3. What are the values of the graduations (unmarked lines) in each of the
following graduated cylinders?
Q
(A)
50
3
graduations =
(B)
50
-40
-30
-20
10
graduations =
(C)
25
-20
-15
10
5
graduations =
Answers
The values of the Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
Graduated Cylinder - A popular piece of scientific equipment used to measure the volume of a liquid is a graduated cylinder, sometimes referred to as a measuring cylinder or mixing cylinder. Its form is slender and cylindrical. Each marked line on the graduated cylinder indicates the volume of liquid that has been measured.
The clear graduated cylinder is widely used for volume measurements and is regarded to be more accurate than a beaker since it contains permanently marked incremental graduations.
To calculate the value of graduations-
1. Subtract the two values to get the value between the labeled graduations.
2. Determine how many spaces there are between the two graduations. Keep in mind that volume equals space.
3. Divide the number of gaps by the value between the graduations.
For cylinder A.
2 ml- 1ml = 1ml
Number of spaces = 5
∴1/ 5 = 0.2
Graduation = 0.2ml
For cylinder B -
20-10 = 10
number of spaces = 5
∴10/ 5 =2
Graduation = 2ml
For cylinder C-
10-5 = 5
Number of spaces= 5
∴5/5 = 1
Graduation = 1ml
Hence , Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
To learn more about graduated cylinders refer-https://brainly.com/question/26216613
#SPJ9
Your question is incomplete. Please find the missing image below.
Pa help po thanks! I need the answer ASAP

Answers
Answer:
1.T
2.I
Explanation:
hope it help
#carry on learning
The object and description that matches is Object 2 and T.
Object 1 has no matching description.
Fish Aquarium FilterA Fish aquarium filter is a filter whose function is to clean the water of debris, removes the toxic buildup of ammonia and nitrates, and aerates the water so that fish can in a conducive environment and breathe properly
Engine Oil FilterAn engine oil filter is a filter whose function is to filter and remove contaminants that may be present in the engine oil, transmission oil, lubricating oil, or hydraulic oil in order for proper functioning of the engine.
Therefore, the object and description that matches is Object 2 and T.
Object 1 has no matching description.
Learn more about filters and their uses at: https://brainly.com/question/10719424