draw the lewis structure for ccl4 in the window below and then decide if the molecule is polar or nonpolar.
Answers
The Lewis structure for CCl4 is: In this molecule, the central atom is carbon which has 4 valence electrons and chlorine atoms have 7 valence electrons.
The total valence electrons in the molecule will be 32 (4*7 + 4*2).To get the Lewis structure of CCl4, first, we need to draw the atoms and connect them with a single bond. After that, we need to fill the valence electrons. It will be 4 electrons on each of the 4 chlorine atoms and 4 electrons on the carbon atom.Then we will add the remaining valence electrons, which will be 4 (8-4) electrons on the carbon atom to complete its octet.
The Lewis structure of CCl4 will have no lone pairs and it will be tetrahedral in shape with bond angles of 109.5 degrees. The molecule of CCl4 is nonpolar because the shape of the molecule is symmetrical and all the chlorine atoms are arranged in the corners of the tetrahedron with equal dipole moments. Thus, the polarities of all bonds in the molecule will cancel each other, making the molecule nonpolar. The polarity of the molecule depends on the distribution of charges in the molecule, which is determined by the molecular shape. If the dipole moments of all the bonds are not equal, then the molecule will be polar, and if the dipole moments are equal, then the molecule will be nonpolar.
To know more about Lewis structure visit:-
https://brainly.com/question/29603042
#SPJ11
Related Questions
What was the function for this procedure: Addition of ethanol to filtered extract
Answers
The function of this procedure, which involves the addition of ethanol to a filtered extract, is to precipitate and separate compounds of interest from the mixture. Ethanol serves as a precipitating agent, causing specific substances to become insoluble and form solid particles.
The filtered extract refers to the mixture obtained after removing solid impurities. The procedure can be broken down into the following steps:
1. Obtain a filtered extract by separating solid impurities from a liquid mixture.
2. Add ethanol to the filtered extract. The ethanol induces precipitation of the desired compounds.
3. Allow the mixture to settle, and the precipitated compounds will form solid particles.
4. Separate the solid particles from the liquid by methods such as centrifugation or filtration.
The reason for this procedure is that ethanol promotes the separation of specific compounds from the mixture, making it easier to isolate and study them. This is important in various fields such as chemistry, biology, and pharmaceutical research.
Learn more about precipitating agent here:
brainly.com/question/15333702
#SPJ11
suppose 2. mole of reactant b with excess reactant a how much of the product can form
Answers
The amount of product that can result from the reaction of two moles of reactant B with an excess of reactant A is determined by the reaction's stoichiometry and the limiting reactant.
A substance used in a chemical reaction known as a reactant is ingested during the reaction and eventually becomes a component of the end result (s). A chemical reaction begins with reactants, which are then converted into the desired end products. The quantity of reactant used in a reaction can affect the volume of product that is produced, and in some circumstances, using too much reactant can guarantee complete consumption of the other reactant. The target product's yield and reaction rate can both be impacted by the identity, purity, and quantity of each reactant. To guarantee that the reaction goes smoothly and that the desired product is obtained, the reactants must be carefully chosen and handled. Occasionally, the reactants may need.
Learn more about reactant here:
https://brainly.com/question/17096236
#SPJ4
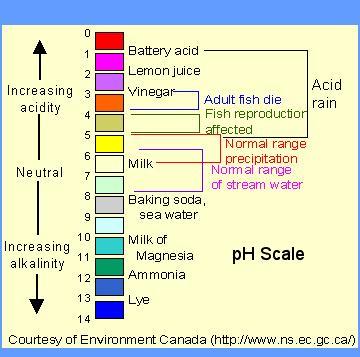
Ph4 is greater than ph6?.
Answers
Answer:
Explanation: For example, pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6. The same holds true for pH values above 7, each of which is ten times more alkaline (another way to say basic) than the next lower whole value.
HOPE THAT HELPS
Electromagnetic waves used in broadcasting are called?
Answers
Un compuesto ZO2 tiene masa molar igual a 64. La masa atómica de Z debe ser: A . 6. C. 12. E. 32. B. 10. D. 24.
Answers
Answer:
32. Opcion E
Explanation:
ZO₂ podemos entenderlo como dióxido de Z
1 mol de ZO₂ contiene 1 mol de Z y 2 moles de O₂ por lo que si la masa molar es de 64 g/mol, podemos plantear lo siguiente
Masa de Z + 2 masa de O = 64 g/mol
Sabemos que la masa del oxígeno es 16 g/mol
x + 2. 16 g/mol = 64 g/mol
x = 64 - 32 → 32 g/mol
La masa atómica coincide con la masa molar, por lo tanto la masa atomica de Z es 32.
Considerando la definición de masa molar, la respuesta correcta es la opción E: La masa atómica de Z debe ser 32.
La masa molar es la masa de un mol de una sustancia, la cual puede ser un elemento o un compuesto, esto es, es la relación entre la cantidad de sustancia y su masa.
En otras palabras, la masa molar es la cantidad de masa que una sustancia contiene en un mol.
En la tabla periódica, se puede encontrar la masa molar de los elementos, también llamada masa atómica o peso atómico.
Para calcular la masa molar de un compuesto, también llamado masa o peso molecular, se debe sumar la masa molar de los elementos del compuesto multiplicado por su cantidad en el compuesto.
Entonces, en este caso, siendo la masa molar del oxígeno (O) 16, y la masa molar del compuesto ZO₂ 64, y denominando x a la masa molar de Z, entonces la masa molar del compuesto puede expresarse como:
x + 2×16= 64
Resolviendo:
x + 32= 64
x=64 -32
x=32
Finalmente, la respuesta correcta es la opción E: La masa atómica de Z debe ser 32.
Aprenda más:
https://brainly.com/question/23183573?referrer=searchResultsHow much silver was in the solution if all of the silver was removed as Ag metal by electrolysis for 0.40 hr with a current of 1.00 mA (1 mA = 10-3 A)?
Answers
The amount of silver that was in the solution before electrolysis was 1.61 x 10^{-3} g.
How does electrolysis work?When an electric current is sent through a substance, electrolysis, a chemical reaction, takes place. As a substance undergoes a chemical reaction, an electron is either gained or lost.
In order to respond to this query, we must apply Faraday's law of electrolysis, which has the following equation:
moles of substance = (electric charge / Faraday's constant)
where the Faraday's constant, which equals 96,485 C/mol e-, measures the amount of electric charge per mole of electrons.
Now, we want to find the amount of silver
Calculate the amount of electric charge;
electric charge = current x time
electric charge = 0.001 A x (0.40 hr x 3600 s/hr) = 1.44 C
Using Faraday's constant, convert the electric charge to moles of electrons:
moles of electrons = electric charge / Faraday's constant
= 1.44 C / 96,485 C/mol e-
= 1.49 x 10^{-5} mol e-
moles of Ag+ = moles of electrons = 1.49 x 10^{-5} mol
The mass of Ag present initially:
mass of Ag = moles of Ag+ x molar mass of Ag
= 1.49 x 10^{-3} mol x 107.87 g/mol
= 1.61 x 10^{-3} g
To know more about the electrolysis visit:
https://brainly.com/question/12994141
#SPJ1
Jason shot a bb straight up in the air with a velocity of 105 m/s.what will the velocity of the bb when it is at a height of 203 m?
Answers
Answer:
The velocity of the bb when it reaches a height of 203 m can be determined using the laws of projectile motion. Since the bb is moving vertically upwards, its velocity at that height will be zero.
brainlest?
Answer: v = 83.96 m/s
Assuming the acceleration due to gravity is approximately 9.8 m/s^2, we can use the principles of projectile motion and energy conservation.
Using the equation for the vertical displacement of an object in free fall:
Δy = (v₀² - v²) / (2g)
Δy = vertical displacement (203m)
v₀ = initial velocity (105 m/s)
v = final velocity (not known yet)
g = accerlation due to gravity (9.8 m/s^2)
Lets rearrange the equation to solve for the final velocity:
v = v = √(v₀² - 2gΔy)
Substituting the given values:
v = √(105² - 2 * 9.8 * 203)
v ≈ √(11025 - 3979.6)
v ≈ √(7054.4)
v ≈ 83.96 m/s
Therefore, when the BB pellet is at the height of 203m, its velocity will be approximately 83.96 m/s.
PLS HELP!! ASAP!! 10 POINTS!!!
Round all values to the nearest whole number.
Imagine you're looking at calcium-43 now, which is not found on the periodic table.
Ca-43 has protons, neutrons, and electrons.

Answers
neutrons : 43
electrons : 86
Why do cells prefer purine and pyrimidine salvage pathways over the de novo synthesis pathway?
Answers
The cells prefer purine and pyrimidine salvage pathways over the de novo synthesis pathway because of their short act of enzymatic reactions.
What are salvage pathways?The salvage pathway is a set of acts or consequences of enzymatic or catalytic reactions to form the rapid products.
In the salvage pathway the enzymes which are used help purine and pyrimidine base pairs to transfer the electrons for metabolism.
Therefore, because of its short act of enzymatic reactions cells prefer purine and pyrimidine salvage pathways over the de novo synthesis pathway.
Learn more about salvage pathways, here;
https://brainly.com/question/28222638
#SPJ4
Please help me answer this question. 3H₂C2O4+2K₂MnO4 → 6CO₂ +2K₂O + Mn₂O3 + 3H₂O
Answers
This is a redox reaction where the C₂O₄ is being reduced to CO₂ and the K₂MnO₂ is being oxidized to Mn₂O₂. The overall reaction is the reduction of C₂O₂ to CO₂ and the oxidation of K₂MnO₂ to Mn₂O₂.
Redox response definition:
Redox (reduction-oxidation) is a chemical reaction that happens when the oxidation states of the substrate change. Losing electrons or raising an element's oxidation state is the process of oxidation. When an element or one of its atoms obtains electrons or experiences a fall in oxidation status, reduction can take place.
There are two types of redox reactions and electron transfers, in which one electron normally goes from the reducing agent to the oxidant. Two terminology that are frequently used to describe this kind of redox reaction are electrode potentials and redox couples. Atom transfer is the transfer of an atom from one substrate to another.
This is a redox reaction.
The half-reactions are:
Oxidation: 3H₂C2O4 + 2K₂MnO₄ → 6CO₂ + 2K₂O + 2Mn₂O₃ + 3H₂O
Reduction: 2MnO₄⁻ + 16H⁺ + 6e⁻ → 2Mn⁺² + 8H₂O
The overall reaction is the sum of the oxidation and reduction half-reactions.
To learn more about redox reaction
https://brainly.com/question/21851295
#SPJ10
Question 22 Cr is a member of which family? noble gases halogens alkaline earth metals alkali metals None of these
Answers
Chromium (Cr) is a member of transition metals family, so the correct answer is E none of these.
What is transition metal?Usually transition metals are strong and hard elements. Both the melting and boiling points of these metals are high. Transitional metals family are versatile materials that can be hammered, bent, and drawn into wires. They also carry heat and electricity effectively. These metals' atoms have a strong attraction to one another. They are typically glossy as well.
The chemical element chromium has the atomic number 24 and the symbol Cr. It is the group 6's first element. It is a transition metal family that is steely-grey, glossy, tough, and brittle.
The great corrosion resistance and hardness of chromium metal make it valuable. The discovery that steel could be rendered extremely resistant to corrosion and discoloration by adding metallic chromium to make stainless steel was a significant advancement in the production of steel. 85% of commercial use is made up of stainless steel with chrome plating (electroplating with chromium). Chromium is highly prized for its ability to be finely polished while remaining tarnish-resistant. Nearly 70% of the visible spectrum and approximately 90% of infrared light are reflected by polished chromium.
Learn more about transition metal at https://brainly.com/question/12843347
#SPJ4
How is heat transferred between substances or systems, and how is it quantified?
Answers
Answer:
Heat can travel from one place to another in three ways: Conduction, Convection and Radiation. Both conduction and convection require matter to transfer heat.
If there is a temperature difference between two systems heat will always find a way to transfer from the higher to lower system.
write a structural formula for each of the following acids: a. 4-oxohexanoic acid b. 2-hydroxy-3-methylhexanoic acid c. 2-chloropentane dioic acid d. p-bromophenyl acetic acid
Answers
4-oxohexanoic acid: CH3(CH2)3COCH2COOH
2-hydroxy-3-methylhexanoic acid: CH3CH(OH)CH(COOH)(CH3)CH2CH2COOH
2-chloropentane dioic acid: ClCH2CH2CH2CH2COOH
p-bromophenyl acetic acid: BrC6H4CH2COOH
4-oxohexanoic acid: The structural formula for 4-oxohexanoic acid can be written as CH3(CH2)3COCH2COOH. This compound has a six-carbon chain with a carbonyl group (C=O) at the fourth position from the carboxylic acid group (COOH). The CH3(CH2)3 part represents the pentyl group attached to the carbonyl carbon, and COOH represents the carboxylic acid group.
b. 2-hydroxy-3-methylhexanoic acid: The structural formula for 2-hydroxy-3-methylhexanoic acid can be written as CH3CH(OH)CH(COOH)(CH3)CH2CH2COOH. This acid contains a six-carbon chain with a hydroxyl group (-OH) attached to the second carbon atom. The CH3CH(OH) part represents the hydroxyethyl group attached to the second carbon atom, and CH(COOH)(CH3)CH2CH2COOH represents the remaining four-carbon chain with a carboxylic acid group at the third position.
c. 2-chloropentane dioic acid: The structural formula for 2-chloropentane dioic acid is ClCH2CH2CH2CH2COOH. This acid has a five-carbon chain with a chlorine atom (Cl) attached to the second carbon atom. The COOH group represents the carboxylic acid group at the end of the chain.
d. p-bromophenyl acetic acid: The structural formula for p-bromophenyl acetic acid is BrC6H4CH2COOH. This acid consists of a phenyl ring (C6H5) with a bromine atom (Br) attached to the fourth position (para position). The CH2COOH group represents the acetic acid group attached to the phenyl ring.
Learn more about: Structural formula
brainly.com/question/29154542
#SPJ11
13. The idea that all matter consists of tiny particles in constant motion is an example of a (n)
Answers
Answer:
An example is the kinetic theory of matter.
According to this theory, all matter consists of tiny particles that are in constant motion.
Particles move at different speeds in matter in different states.
What is the specific heat capacity of an unknown metal if 75.00 g of the metal absorbs 418.6J of heat and the temperature rises 25.0°C?
Answers
Answer:
The specific heat capacity of the unknown metal is 0.223 \(\frac{J}{g*C}\)
Explanation:
Calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
There is a direct proportional relationship between heat and temperature. The constant of proportionality depends on the substance that constitutes the body as on its mass, and is the product of the specific heat by the mass of the body. So, the equation that allows calculating heat exchanges is:
Q = c * m * ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case, you know:
Q= 418.6 J c= ? m= 75 g ΔT= 25 CReplacing:
418.6 J= c* 75 g* 25 C
Solving:
\(c=\frac{418.6 J}{75 g*25 C}\)
c= 0.223 \(\frac{J}{g*C}\)
The specific heat capacity of the unknown metal is 0.223 \(\frac{J}{g*C}\)
Guided
Learning
ar Inequalities in One Variable - Item 7601
Pre-Quiz
Practice
from a, it will be greater than b.
Post-Quiz
Finish
If the ordered pair (a, b) satisfies the inequality y> x-4, three of these statements are
true. Which statement is NOT true?
a and b may be equal to each other.
We
Answers
The statement about the inequality that is NOT true is (A), a and b may be equal to each other.
How to determine true statements?This is because the inequality y> x-4 means that y must be greater than x-4. If a and b are equal, then y = x-4, which means that the inequality is not satisfied.
The other three statements are true because:
If 4 is subtracted from a, it will be greater than b because y> x-4 means that y must be greater than x.
If 4 is subtracted from b, it will be less than a because y> x-4 means that y must be greater than x.
If 4 is subtracted from both a and b, the inequality will still be true because y> x-4 means that y must be greater than x, even if x and b are both decreased by 4.
Therefore, the answer to the question is the statement a and b may be equal to each other.
Find out more on inequality here: https://brainly.com/question/25275758
#SPJ1
Complete question:
Guided Learning ar Inequalities in One Variable - Item 7601
Pre-Quiz
Practice
from a, it will be greater than b.
Post-Quiz
Finish
If the ordered pair (a, b) satisfies the inequality y> x-4, three of these statements are true. Which statement is NOT true?
a and b may be equal to each other.
If you subtract 4 from a, it will be greater than b.
If you subtract 4 from b, it will be less than a.
If you subtract 4 from both a and b, the inequality will still be true.
Press Heat to heat up the water. Wait until the temperature
stops rising and observe. What happens?
Answers
The water evaporates
Explanation:
Water is supposed to evaporate when heat is added to it
I believe that it will turn into water vapor
Explanation:
5. A solution has a pH of 9. The solution is best described as:
Answers
Answer: Basic
Explanation:
A solution with a pH of more than 7 is considered basic.
In a first-order decomposition reaction, 50.0% of a compound decomposes in 13.0 min. What is the rate constant of the reaction?
Answers
The rate constant of the reaction is 0.0531 min-¹.For a first-order reaction, the rate of the reaction is proportional to the concentration of the reactant. The rate law for a first-order reaction is given by the equation:
Rate = k[A]
where:
Rate is the rate of the reaction
k is the rate constant
[A] is the concentration of the reactant
The integrated rate law for a first-order reaction is:
ln([A]t/[A]0) = -kt
where:
[A]t is the concentration of the reactant at time t
[A]0 is the initial concentration of the reactant
k is the rate constant
t is the time
In this problem, we are given that 50.0% of a compound decomposes in 13.0 min. This means that [A]t/[A]0 = 0.5, and t = 13.0 min. Substituting these values into the integrated rate law, we get:
ln(0.5) = -k(13.0 min)
Solving for k, we get:
k = -ln(0.5)/13.0 min
k = 0.0531 min-¹
Therefore, the rate constant of the reaction is 0.0531 min-¹
To learn more about rate constant here:
https://brainly.com/question/20305871
#SPJ11
What do each of the variables in Coulomb’s law stand for and what are their units?
Answers
Answer:
File down there
Explanation:

Which statement correctly describes extreme weather?
O A. Extreme weather events follow normal climate patterns.
o O B. Extreme weather events are random occurrences.
O C. Extreme weather events started with global warming.
O D. Extreme weather events are unpredictable until they strike.
Answers
Answer: A
Explanation:
Extreme weather events follow normal climate patterns.
Write a net ionic equation to show that ethylamine, C2H5NH2 behaves as a Bronsted-Lowry base in water. (For organic molecules enter elements in order they are given in the question.) Write a net ionic equation to show that benzoic acid, C6H5COOH, behaves as a Bronsted-Lowry acid in water.
Answers
The net ionic equation for the behavior of ethylamine (C₂H₅NH₂) as a Bronsted-Lowry base in water is:
C₂H₅NH₂ + H₂O → C₂H₅NH₃⁺ + OH⁻
The net ionic equation for the behavior of benzoic acid (C₆H₅COOH) as a Bronsted-Lowry acid in water is:
C₆H₅COOH + H₂O → C₆H₅COO⁻ + H₃O⁺
In water, ethylamine (C₂H₅NH₂) can act as a Bronsted-Lowry base by accepting a proton (H⁺) from water. The reaction can be represented by the net ionic equation: C₂H₅NH₂ + H₂O → C₂H₅NH₃⁺ + OH⁻. In this equation, ethylamine (C₂H₅NH₂) accepts a proton from water (H₂O) to form the ethylammonium ion (C₂H₅NH₃⁺) and hydroxide ion (OH⁻). This shows the base behavior of ethylamine as it accepts a proton.
On the other hand, benzoic acid (C₆H₅COOH) can act as a Bronsted-Lowry acid in water by donating a proton (H⁺) to water. The reaction can be represented by the net ionic equation: C₆H₅COOH + H₂O → C₆H₅COO⁻ + H₃O⁺.
In this equation, benzoic acid (C₆H₅COOH) donates a proton to water (H₂O) to form the benzoate ion (C₆H₅COO⁻) and hydronium ion (H₃O⁺). This demonstrates the acid behavior of benzoic acid as it donates a proton.
To know more about Bronsted-Lowry acid refer here:
https://brainly.com/question/29317749#
#SPJ11
A 295 g aluminum engine part at an initial temperature of 13.00 degrees C, absorbs 75.0 kJ of heat. What is the final temperature of the part
Answers
At a starting temperature of 13.00 degrees Celsius, a 295 g aluminium engine component absorbs 75.0 kJ of heat. The component's ultimate temperature is 296.7 °C.
The following equation can be used to solve this problem:
Q is the amount of heat absorbed, m is the mass of the aluminium component, c is the material's specific heat, and T is the temperature change.
We are aware that the starting temperature is 13.00 degrees C, Q = 75.0 kJ, and m = 295 g. Aluminium has a specific heat of 0.902 J/g°C, which may be found by looking it up.
In the beginning, we must change the mass into kilogrammes and the heat into joules:
m = 0.295 kg
Q = 75.0 kJ = 75,000 J
The equation may now be rearranged to account for ΔT:
ΔT = Q / (mc)
ΔT = 75000 J/(0.295 kg x 0.902 J/g °C)
ΔT=283.7 degrees Celsius
By multiplying the beginning temperature by the temperature change, we can finally determine the final temperature:
T final = 13,000 °C plus 283,7 °C.
T final = 296.7 °C
As a result, the aluminium part's final temperature is 296.7 °C.
Learn more about specific heat here
https://brainly.com/question/28302912
#SPJ11
Life cycles are:
random
divided into two stages
predictable
exactly the same for all species
Answers
The half-life of cobalt-60 is 5. 20 yr. how many milligrams of a 2. 000 mg sample remain after 6. 55 years?
Answers
0.84 milligrams of a 2. 000 mg sample remain after 6. 55 years, according to radioactive decay.
Given data,
\(t\frac{1}{2} of Co-60 = 5.20years\)
amount of sample = 2.000mg initially = 0.002grams
According to radioactive decay,
\(N_{t} = N_{0}e^{-λt}\)
(\(N_{0} - 0.002\) )λ = \(\frac{0.693}{t\frac{1}{2} }\) = \(\frac{0.693}{5.20}\) = 0.133
According to radioactive decay,
\(N_{t} = N_{0}e^{-λt}\)
\(lnN_{t} = lnN_{0}\) - λt
\(lnN_{t}\) = ln0.002 - (0.133×6.55)
= -6.21 - 0.87 = -7.08 = 0.00084g = 0.84mg
Therefore, 0.84 milligrams of a 2. 000 mg sample remain after 6. 55 years.
Learn more about radioactive decay here:
https://brainly.com/question/1770619
#SPJ4
Which subatomic particle(s) are located within the nucleus of an atom?
Answers
Protons and neutrons are present inside the nucleus of an atom while electrons revolve around circular orbits.
What is Proton and Neutrons?
The atom is made up of the subatomic particles electron, proton, and neutron. The atom is made up of a core nucleus that has neutrons and protons in it. The nucleus is surrounded by electrons.
Neutrons are neutral, protons are positively charged, and electrons are negatively charged.
All atoms' nuclei include protons, which are subatomic particles with a positive charge. One proton has a positive charge. The quantity of protons in the element's nucleus determines its atomic number. Two up quarks and one down quark make up protons. The mass of it is 1.007277 amu (atomic mass units).
All atoms, with the exception of hydrogen, include neutrons, which are subatomic particles that are neutrally charged.
Learn more about Proton and Neutron from given link
https://brainly.com/question/25674345
#SPJ1
A glass column is filled with mercury and inverted in a pool of mercury. The mercury column stabilizes at a height of 729 mm above the pool of mercury. What is the pressure of the atmosphere
Answers
The atmospheric pressure will be:
The pressure of the atmosphere resulting from the mercury column is 0.959 atm
What is atmospheric pressure?
The force that an object experiences from the weight of the air above it per unit area are known as atmospheric pressure.
Given: Height of mercury column = 729 mm Hg
To find: The pressure of the atmosphere
Calculation:
The atmospheric column resulting from the mercury column is calculated as follows:
1 atm =760 mm Hg
So, we can convert the 729 mm Hg to atm, and we get
Atmospheric pressure = 729 x 1 atm / 760 = 0.959 atm
Learn more about atmospheric pressure here,
https://brainly.com/question/14315894
#SPJ4
Is soap necessary to remove salt (NaCl) from your hands? Explain
Answers
No, soap isn't necessary to remove salt from hands.
SoapSoap is an amphipathic substance, that is, it has a polar part and a non-polar part.
In view of this, this substance is very necessary for cleaning hands when they are dirty with something nonpolar, such as oil, as it can bind to the dirt molecules and leave in the water.
In the case of salt, as it is not a non-polar substance, just water is enough to clean salt from your hands.
Learn more about saponification and soap in: brainly.com/question/14018257
identify the first step in preparing a spectrophotometer for use.
Answers
We have that the first step is to put the Power source into an on state,Thereby powering the Light point and the Spectrophotometer.
From the question we are told
identify the first step in preparing a spectrophotometer for use.
Generally
A SpectrophotometerThis is a device is out there to help scientist i the mostly in the field of chemistry.
This device is used to Know or arcertain particle with light consuming properties.
The Spectrophotometer is Mostly found in laboratories.
And usually in the use of a Spectrophotometer the first step is to put the Power source into an on state.Thereby powering the Light point and the Spectrophotometer
Therefore
The first step is to put the Power source into an on state,Thereby powering the Light point and the Spectrophotometer.
For more information on this visit
https://brainly.com/question/14379882
1) A 45.7 g sample of glass was brought to thermal equilibrium with boiling water and then transferred to 250.0 g of water that was at 22.5 °C. This combination reached thermal equilibrium at 24.2 °C. What is the specific heat capacity of glass?
2) What mass of silver at 100.0 °C, when added to 150.0 g of water at 21.0 °C, would raise the water’s temperature to 24.0 °C ?
3) The molar heat of fusion of H2O is 6.1 kJ/mole. What amount of energy would be needed to melt 376 g of ice at 0.0 °C?
Answers
1) A 45.7 g sample of glass was brought to thermal equilibrium with boiling water and then transferred to 250.0 g of water that was at 22.5 °C. This combination reached thermal equilibrium at 24.2 °C. What is the specific heat capacity of glass?
2) What mass of silver at 100.0 °C, when added to 150.0 g of water at 21.0 °C, would raise the water’s temperature to 24.0 °C ?
3) The molar heat of fusion of H2O is 6.1 kJ/mole. What amount of energy would be needed to melt 376 g of ice at 0.0 °C?