Answers
Answer:
C=37.5% H=4.2% O=58.3%
Explanation:
molar mass of element / molar weight of compound X 100% (for each element)
C mass = 12 x 6 = 72
H mass = 1 x 8 = 8
O mass =16x 7 = 112
Molar weight of compound = 192
72/192(100%)= 37.5% C
8/192(100%) = 4.2 % H
112/192 (100%) = 58.3% O
The mass percentage of carbon, hydrogen, H, and oxygen, O, in C₆H₈O₇ is C = 37.5% H = 4.2% O = 58.3%.
What is the mass percentage?The concentration of an element in a compound or component in a combination is calculated by the mass percentage.
The collective of a component is divided by the entire mass of the mixture to obtain the mass percentage, which is then multiplied by 100%.
Given, finding the mass percentage of carbon, hydrogen, H, and oxygen, O, in C₆H₈O₇
the molar mass of element / molar weight of compound X 100% (for each element
First, calculate the molar mass of the elements given in the compound.
C mass = 12 x 6 = 72
H mass = 1 x 8 = 8
O mass =16x 7 = 112
Molar weight of compound = 192
72/192(100%)= 37.5% C
8/192(100%) = 4.2 % H
112/192 (100%) = 58.3% O
Thus, the mass percentage is C = 37.5% H = 4.2% O = 58.3%.
To learn more about mass percentage, refer to the below link:
https://brainly.com/question/16885872
#SPJ2
Related Questions
Which of the following processes absorbs energy? A) condensation of water on a wind shield of a car B) formation of copperC) ball rollinflown a hill D) formation of ice from liquid water
E) oxide from copper and oxygen
Answers
Answer:
C) ball rollinflown a hill
Explanation:
The question asks to identify the endothermic process in the list of options. By way of elimination, we have;
A) condensation of water on a wind shield of a car
Condensation is an exothermic process. That is, heat is given out as the gases change into the liquid state of matter.
B) formation of copper
This is an exothermic process. Capture of electrons by a cation is always exothermic.
C) ball rollinflown a hill
This is the correct option. Energy is absorbed by the ball as it moves on the hill
D) formation of ice from liquid water
Freezing is an example of exothermic reaction. Heat is given off to the surroundings.
E) oxide from copper and oxygen
Formation of metal oxides and most reactions involving oxygen are exothermic reactions,
Which of the following does NOT influences climate change?
Which of the following does NOT influences climate change?
All of these have an effect on climate change
Planting trees
Burning greenhouse gases
driving cars
Answers
Answer:
Driving cars
Explanation:
Because driving cars won't effect climate change
50 points, and I’ll mark as brainliest!!!!!
Tasks are in the picture.

Answers
pH determines the acidic or alkaline a solution is using the pH scale, which has a range of 0 to 14. An alkaline pH is greater than 7, while an acidic pH is less than 7.
Thus, The pH of a solution is defined mathematically as the negative logarithm of the molar concentration of hydrogen ions therein.
NaOH is a strong alkaline, as indicated by a pH testing strip, but in order to determine its exact pH, you must first determine its molarity.
A scale known as pH is used to describe how basic or acidic a water-based solution is. Basic solutions have a higher pH than acidic solutions, which have a lower pH.
Thus, pH determines the acidic or alkaline a solution is using the pH scale, which has a range of 0 to 14. An alkaline pH is greater than 7, while an acidic pH is less than 7.
Learn more about pH, refer to the link:
https://brainly.com/question/15289741
#SPJ1
The pH of HNO₂ is 2.15, pH of NH₄OH is 10.98 and pH of H₂S is 3.76.
pH is defined as the negative logarithm of H⁺ ion concentration.
pH is a measure of how acidic or basic a substance is. In our everyday routine, we encounter and drink many liquids with different pH. Water is a neutral substance. Soda and coffee are often acidic.
The pH is an important property, since it affects how substances interact with one another and with our bodies. In our lakes and oceans, pH determines what creatures are able to survive in the water.
Given,
1. Concentration = 0.1
Ka = 4.5 × 10⁻⁴
\(pH = \frac{1}{2} (pka - log c)\)
pH = 0.5 × ( 3.3 + 1)
= 2.15
2. Concentration = 0.05
Ka = 1.8 × 10⁻⁵
\(pOH = \frac{1}{2} (pkb - log c)\)
pOH = 0.5 × ( 4.74 + 1.3)
= 3.02
pH = 14 - pOH
= 14 - 3.02
= 10.98
3. Concentration = 0.3
Ka = 1 × 10⁻⁷
\(pH = \frac{1}{2} (pka - log c)\)
pH = 0.5 × ( 7 + 0.52)
= 3.76
Learn more about pH, here:
https://brainly.com/question/15289714
#SPJ1
An organic molecule contains 2.088 Liters Carbon, 2.688 Liters Hydrogen, and 0.896 Liters oxygen. Calculate the Empirical formula.
Answers
The empirical formula of the organic molecule, given that it contains 2.088 L Carbon, 2.688 L Hydrogen and 0.896 L oxigen is C₃H₄₈O
How do I determine the empirical formula?The following data were obtained from the question:
Carbon (C) = 2.088 LHydrogen (H) = 2.688 LOxygen (O) = 0.896 LEmpirical formula =?The empirical formula of the organic compound can be obtained as follow:
Divide by their molar mass
C = 2.088 / 12 = 0.174
H = 2.688 / 1 = 2.688
O = 0.896 / 16 = 0.056
Divide by the smallest
C = 0.174 / 0.056 = 3
H = 2.688/ 0.056 = 48
O = 0.056 / 0.056 = 1
Thus, we can conclude that the empirical formula of the organic molecule is C₃H₄₈O
Learn more about empirical formula:
https://brainly.com/question/9459553
#SPJ1
Question in picture
Question in picture

Answers
The correct answer is a sphybridisation in z coordinate.So to form sphybridisation we need a s orbital and a p orbital .
In genomics, hybridization is the process by which two complementary single-stranded DNA or RNA molecules bond together to form a double-stranded molecule.The bonding is determined by the correct base pairing between the two single-stranded molecules. When one s and one p orbital in the same main shell of an atom combine to form two new equivalent orbitals, this is referred to as sphybridization.
The newly formed orbitals are known as sphybridized orbitals. It forms linear molecules with a 180° angle. Atomic orbitals include both s and p orbitals. These orbitals represent the most likely region in which we can find an electron of that atom. The primary distinction between s and p orbitals is that s orbitals are spherical in shape, whereas p orbitals are dumvell-shaped.So to form sp hybridisation we need a s orbital and a p orbital .
Learn more about hybridisation here :-
https://brainly.com/question/14140731
#SPJ9

As a result of the particles in a gas being in constant motion, gas has a _______.
variable volume
variable Pressure
variable Shape
variable mass
Answers
Answer:
i think it's variable pressure
if not soo advance sorry :)
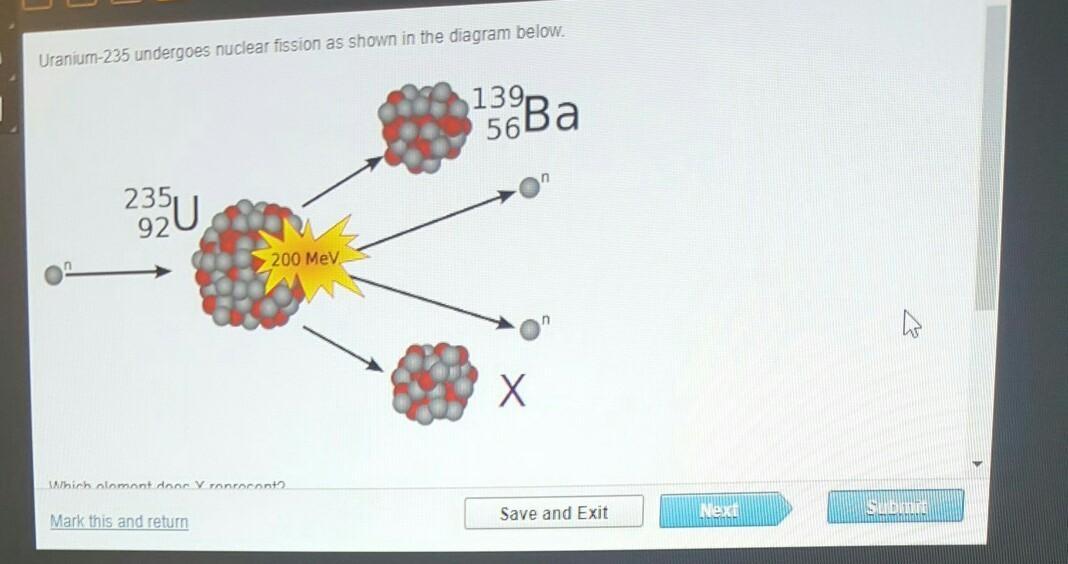
Uranium-235 undergoes nuclear fission as shown in the diagram below. A small gray ball labeled n impacts a large mass of red and gray balls labeled superscript 235 subscript 92 upper U. The impact gives off a smaller mass of balls labeled superscript 139 subscript 56 upper B a, a starburst labeled 200 megavolts, which in turn gives off 2 balls labeled n; and another smaller mass of balls labeled X. Which element does X represent? Superscript 95 Subscript 36 Baseline Upper K r Superscript 96 Subscript 36 Baseline Upper K r Superscript 97 Subscript 36 Baseline Upper K r Superscript 98 Subscript 36 Baseline Upper K r
Answers
Answer:
95/36 Kr or A
Explanation:
In the given nuclear fission reaction, X represents ⁹⁵₃₆Kr. Therefore, the correct option is option A.
What is nuclear fission?Nuclear fission is the splitting of a nucleus of heavy atoms, such that of plutonium or uranium, into two roughly equal mass fragments. A significant amount of energy is released during the process. The core of an atom splits into two nuclei that are lighter during nuclear fission.
When the nucleus is excited by a variety of particles (such as neutrons, protons, deuterons, and alpha particles) or by electromagnetic radiation takes the form of gamma rays, the process may occur spontaneously or be induced. In the given nuclear fission reaction, X represents ⁹⁵₃₆Kr.
Therefore, the correct option is option A. In the given nuclear fission reaction, X represents ⁹⁵₃₆Kr.
To know more about nuclear fission, here:
https://brainly.com/question/29141330
#SPJ3
Your question is incomplete but most probably your full question was,
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
which proess is part of the carbon cycle
Answers
Answer:
The key processes in the carbon cycle are: carbon dioxide from the atmosphere is converted into plant material in the biosphere by photosynthesis.
Explanation:
organisms in the biosphere obtain energy by respiration and so release carbon dioxide that was originally trapped by photosynthesis. ... The carbon becomes part of the .
What did James Cameron use on his 2nd visit to this famous ship to look inside?
Answers
Answer:
beneath the surface of the Pacific Ocean comes from samples and video collected by an unmanned lander,
How much heat will be absorbed by a 26.3 g piece of aluminum (specific heat = 0.930 J/g・°C) as it changes temperature from 23.0°C to 67.0°C?
Answers
Answer:
\(\boxed {\boxed {\sf 1076.196 \ Joules}}\)
Explanation:
Since we are given the mass, specific heat, and temperature, we should use the following formula for heat energy.
\(q=mc\Delta T\)
The mass of the aluminum is 26.3 grams. Its specific heat is 0.930 Joules per gram degree Celsius. We need to find the change in temperature.
The change in temperature is the difference between the initial temperature to the final temperature. The temperature changes from 23.0°C to 67.0°C, so the initial is 23 degrees and the final is 67 degrees. ΔT= final temperature - initial temperature ΔT= 67°C - 23°C ΔT= 44°CNow we know all the values.
m= 26.3 g c= 0.930 J/g °C ΔT= 44°CSubstitute the values into the formula.
\(q= (26.3 \ g}) \times (0.930 \ J/g \textdegree C) \times (44 \textdegree C)\)
Multiply the first two numbers together. The units of grams cancel.
\(q= 24.459 \ J/ \textdegree C \times 44 \textdegree C\)
Multiply again. This time, the units of degrees Celsius cancel.
\(q=1076.196 \ J\)
1076.196 Joules of heat will be absorbed by the piece of aluminum.
A small amount of chemical splashes in Frank’s eye. What should Frank do immediately?
Answers
Answer:
A small amount of chemical splashes in Frank's eye. What should happen next? Frank should go to the eyewash station while his lab partner tells the teacher what happened.
Explanation:
Brainlist
11. Asexual reproduction has certain advantages. This sort of reproduction needs only one parent and can occur by budding and fragmentation. What is an advantage of sexual reproduction over asexual reproduction? A. exact copy of the genes B. reproduce quickly C. greater genetic variation D. finding a mate
Answers
Answer:
C. Greater genetic variation
CuI2 (light brown solid) name copper compounds
Answers
CuI2 is not a known compound. Copper compounds typically have different oxidation states for copper, resulting in various compound names.
Copper(II) oxide (CuO): It is a black solid compound where copper is in the +2 oxidation state. It is commonly used as a pigment and in catalytic reactions.
Copper(II) sulfate (CuSO4): It is a blue crystalline compound in which copper is in the +2 oxidation state. It is used in various applications such as agriculture, electroplating, and as a laboratory reagent.
Copper(I) oxide (Cu2O): It is a red crystalline compound in which copper is in the +1 oxidation state. It is used as a pigment, in solar cells, and as a catalyst.
Copper(II) chloride (CuCl2): It is a greenish-brown solid compound in which copper is in the +2 oxidation state. It is utilized in various chemical processes, including etching and catalyst synthesis.
Copper(II) nitrate (Cu(NO3)2): It is a blue crystalline compound where copper is in the +2 oxidation state. It is commonly used in the production of catalysts, as a coloring agent, and in electroplating.
These are just a few examples of copper compounds with different oxidation states and properties. It's important to note that the compound CuI2 mentioned in the question, if it exists, would be an exception to the typical nomenclature for copper compounds.
For more such questions on oxidation visit:
https://brainly.com/question/13182308
#SPJ8
A sample of chlorine has two naturally occuring isotopes, the isotope Cl-35 makes up 75.8% of the sample, and the isotope cl-37 makes up 24.3 of the sample. Which of the following statements is true?
a. the atomic mass of chlorine will be less than 35
b. the atomic mass will be between 35 and 37
c. you can't tell what the atomic mass will be
d. the atomic mass of clorine will be more thatn 37
Answers
The statements which is true is the atomic mass will be between 35 and 37.
Isotopes can be defined as the tittles of identical rudiments that are composed of the same number of electrons and protons but differ in case of the number of neutrons. Since the number of neutrons is different in colorful isotopes of an element so, their infinitesimal millions also vary. still, the infinitesimal number remains the same as no change occurs in protons number.
Chlorine is an element in the periodic table with infinitesimal number 17 and is represented with the symbol Cl. The element nickel has colorful isotopes. One of the isotopes of chlorine is chlorine- 35 and another bone is chlorine-37 which is present in a rate of 75.8% and24.3%.
The average atomic mass of chlorine;
Average atomic mass
=(Fractional abundance of 35Cl) × (Molar Mass of 35Cl) +(Fractional abundance of 37Cl) × (Molar Mass of 37Cl)
=75.8100 × 35u + 24.3100 × 37u
=26.53u + 8.991u
=35.521 u
The average atomic mass of chlorine is 35.521 u.
Learn more about Isotopes:
https://brainly.com/question/13602441
#SPJ4
*SCIENCE*
A nebula contains large amounts of dust and clouds. What role does gravity play inside of nebulae?
0 The gravitational pull between the gas and dusť matter leads to the formation of new galaxies.
0 The gravity pulls the gas and dust apart, leading to the formation of a larger nebula.
0 The gravitational pull between the gas and dust matter leads to the formation of new stars and planets.
0 There is no gravity inside nebulae because each dust particle is so small.
Answers
Answer:
The gravitational pull between the gas and dust matter leads to the formation of new galaxies
Explanation:
My teacher went over the answers and said that one was correct.
A nebula contains large amounts of dust and clouds. The gravitational pull between the gas and dusť matter leads to the formation of new galaxies does gravity play inside of nebulae. Therefore, option A is correct.
What is gravity ?Gravity is the force that pulls objects toward the center of a planet or other body. The gravitational force keeps all the planets in orbit around the sun.
Gravity pulls you toward the ground because all objects with mass, such as our Earth, actually bend and curve the fabric of the universe, known as spacetime. Gravity is the curvature of the earth.
A nebula is densely packed with dust and clouds.The gravity play a role inside nebulae, The gravitational pull between gas and dus matter leads to the formation of new galaxies.
Thus, option A is correct.
To learn more about the gravity, follow the link;
https://brainly.com/question/4014727
#SPJ6
A flammable gas made up of only carbon is found to effuse through a porous barrier in 1.50min. it takes an equal volume of bromine vapor 4.73min to effuse through the same barrier. Calculate the molar mass of the unknown gas and suggest what
the gas might be
Answers
Methane is the unidentified gas since its molar mass is 16 g/mol.
When describing atomic masses and molecular masses, the Dalton (Da) or unified atomic mass unit (u) are two alternative units of mass that are frequently employed. It is known to be 1/12 the mass of a carbon-12 atom and is also referred to as "amu" in older.
We must be aware that a gas's molar mass directly relates to how long it takes to diffuse.
t1/t2 = √M1/M2
Let t1 equal the 1.50 minutes it takes for the combustible gas to disseminate. Let t2 equal the 4.73 minutes it takes for the bromine vapor to disperse.
M1 = the flammable gas's molar mass equals
160 g/mol is the molar mass of the bromine vapor, or M2.
replacing values.
1.50/4.73 = √M1/160
(1.50/4.73) ^2 = M1/160
0.1006 = M1/160
M1 = 0.1006 × 160
M1 = 16 g/mol
Methane is the gas in question.
Learn more about molar mass here-
https://brainly.com/question/22997914
#SPJ4
A student planned to make copper sulfate crystals from excess copper oxide and dilute sulfuric acid.
The equation for the reaction is:
CuO(s) + H,SO (aq) -, CuSO (aq) + H20(1)
This is the method used.
1. Add 25 cm° of dilute sulfuric acid to a conical flask.
2. Gently warm the dilute sulfuric acid.
3. Add excess copper oxide to the dilute sulfuric acid.
4. Stir the mixture.
5. Heat to evaporate all the water from the mixture.
Suggest two improvements to the method
Explain why each improvement is needed.
A student plans a method to prepare pure crystals of copper sulfate.
The student's method is:
1. Add one spatula of calcium carbonate to dilute hydrochloric acid in a beaker.
2. When the fizzing stops, heat the solution with a Bunsen burner until all the liquid is gone.
The method contains several errors and does not produce copper sulfate crystals.
Explain the improvements the student should make to the method so that pure crystals of copper sulfate are produced.

Answers
The student's method for preparing pure crystals of copper sulfate contains errors and does not produce the desired outcome.
Use copper oxide instead of calcium carbonate: The student should add copper oxide (CuO) to the hydrochloric acid instead of calcium carbonate. Copper oxide reacts with hydrochloric acid to form copper chloride, which can then be converted to copper sulfate through a subsequent reaction with sulfuric acid.
Add sulfuric acid to the copper chloride solution: After the copper chloride solution is formed, the student should add sulfuric acid to it. This reaction between copper chloride and sulfuric acid will yield copper sulfate and hydrochloric acid. The student should ensure that the correct stoichiometric ratio is maintained to maximize the yield of copper sulfate crystals.
Crystal formation: The student should allow the solution to cool slowly after the reaction with sulfuric acid. This promotes the formation of larger, well-defined copper sulfate crystals.
Filtration and drying: Once the crystals have formed, the student should filter the solution to separate the solid crystals from the remaining liquid. The filtered crystals should then be thoroughly dried to remove any remaining water, resulting in pure copper sulfate crystals.
By following these improvements, the student can obtain pure crystals of copper sulfate.
For more such questions on copper sulfate visit:
https://brainly.com/question/17439051
#SPJ8
How many grams of NaOH are needed to make a 250.0 mL or a 4.2 M NaOH solution?
Group of answer choices
A. 1.05 grams NaOH
B. 42,000 grams NaOH
C. 42 grams NaOH
D. 1050 grams NaOH
Answers
Answer:
C.) 42 grams NaOH
Explanation:
To find the mass of NaOH, you need to (1) find the moles (using the molarity equation) and then (2) convert moles to grams (using the molar mass).
(Step 1)
250.0 mL / 1,000 = 0.2500 L
Molarity = moles / volume (L)
4.2 M = moles / 0.2500 L
1.05 = moles
(Step 2)
Molar Mass (NaOH): 22.990 g/mol + 15.998 g/mol + 1.008 g/mol
Molar Mass (NaOH): 39.996 g/mol
1.05 moles NaOH 39.996 g
----------------------------- x ------------------ = 42 grams NaOH
1 mole
3.(a) Draw the following five-carbon hydrocarbons:•Pentane•Pentene•Pentyne(e)Classify each molecule and explain the differences between them. (Classes of organic molecules include alkanes, alkenes, alkynes, alcohols, carboxylic acids, aldehydes, ketones, esters, ethers, amines, and amides.)
Answers
Structural formulas are ways atoms and elements are arranged in a molecule. The structural formula of pentane, pentene, and pentyne are as shown below;
Pentane - C5H12
Pentene - C5H10
Pentyne - C5H8
e) Pentane is an alkane compound with a general formula CnH2n+2. The chemical formula of pentane is C5H12. There are only single bonds between the C-H atoms in the molecule.
Pentene is an alkene compound with a general formula of CnH2n. The chemical formula of pentene is C5H10. There is a double bond present between the C=H atoms. This double bond shows that the compound is an alkene.
Pentyne is an alkyne compound with a general formula of CnH2n-2. The chemical formula of pentene is C5H8. There is a triple bond present between the Carbon Hydrogen atoms. This presence of a triple bond shows that the molecule is an alkyne compound.

Predict the missing component in the nuclear equation.

Answers
The missing component is 23/11 Na
What is the positron emission?Positron emission is a type of radioactive decay that occurs in certain unstable atomic nuclei. During positron emission, a proton in the nucleus is converted into a neutron, and a positively charged particle called a positron is emitted from the nucleus.
Positron emission is used in medical imaging techniques such as positron emission tomography (PET), which involves injecting a small amount of a radioactive substance into the body and detecting the gamma rays produced by the annihilation of positrons with electrons in the body's tissues. This allows physicians to obtain detailed images of the body's internal organs and structures.
Learn more about positron emission:https://brainly.com/question/20388835
#SPJ1
How many grams of magnesium (Mg, 24.30g/mol) are in 7.43 x 102 atoms of Mg?Let's begin by setting up our expression.Which part of the conversion factor shouldgo in the green box?7.43x1022 atoms Mg|[?]A. 6.02 x 1023 atoms MgB. 1 mole MgEnter

Answers
According to the given question, the part of the conversion factor that should go in the green box is the Avogadro's number, which is 6.02x10^23.
It means the correct answer is A.
In the red box that is above the green box, you should put 1 mole Mg.
In the red box that is next to it, you should put 24.30 grams Mg and in the one that is below, 1 mole Mg.
When the conductivity is at a minimum, what must be true about the amount of Ba(OH)2?
Answers
When the conductivity is at a minimum, the true thing about the amount of Ba(OH)₂ is that the amount of the two reactants are the same when conductivity is low.
What is conductivity?The ability of an aqueous solution to carry an electric current is measured by its conductivity. Ionic strength is measured by conductivity monitors. In automated chromatography systems, conductivity is the main input control parameter used to enable the generation of salt gradients or to control buffer dilution or in-line buffer preparation.
Conductivity monitors can be used to automate and monitor cleaning and equilibration procedures. It's important to remember that temperature affects measurements of conductivity and pH.
When conductivity is low, the amounts of the two reactants are equal. Between the reactants, there are no excesses. The aqueous solution cannot dissolve the precipitate BaSO₄. Since there are no ions, the conductivity is at its lowest.
Learn more about conductivity on:
https://brainly.com/question/1851658
#SPJ1
Complete question
The Ba(OH)₂ dissociates as Ba+2 + 2 OH-. H₂SO₄ dissociates as 2 H+ + SO₄-2. When the conductivity is at a minimum, what must be true about the amount of Ba(OH)₂ compared to H₂SO₄
Given the equation: HCI + Na2SO4 ---> NaCl + H2SO4 Hint: you should balance the equation If you start with 2.0 g of HCl (hydrochloric acid), how many grams of H2SO4 (sulfuric acid) will be produced?
Answers
Answer:
approx 2.45g
Explanation:
2HCl + Na2SO4 → 2NaCl + H2SO4
2 : 1 : 2 : 1
0.05 0.025 (moles)
⇒ mH2SO4 = 0.025 × 98 = 2.45 g
What is the total number of peaks due to singly charged ions in the complete mass
spectrum of chlorine, Cl2
?
A Two
B Three
C Four
D Five
Answers
Five is the total number of peaks due to singly charged ions in the complete mass spectrum of chlorine, \(Cl_{2}\)
How many peaks do \(Cl_{2}\)'s molecular ions have?
The mass spectra of compounds with a single chlorine atom show two molecular ion peaks. This is because there are two isotopes of chlorine, 35Cl and 37Cl.
The molecular ion and fragment ions will both have peaks in the mass spectrum. When a mass spectrum is interpreted, a specific molecule can be located, confirmed, or its quantity can be calculated. the base summit of a mass spectrum's tallest (strongest) peak, caused by the ion with the highest relative abundance
To learn more about chlorine atom use:
brainly.com/question/30861877
#SPJ1
Five is the total number of peaks due to singly charged ions in the complete mass spectrum of chlorine, \(Cl_{2}\).
How many peaks do 's molecular ions have?
The mass spectra of compounds with a single chlorine atom show two molecular ion peaks. This is because there are two isotopes of chlorine, 35Cl and 37Cl.
The molecular ion and fragment ions will both have peaks in the mass spectrum. When a mass spectrum is interpreted, a specific molecule can be located, confirmed, or its quantity can be calculated. the base summit of a mass spectrum's tallest (strongest) peak, caused by the ion with the highest relative abundance
To learn more about chlorine atom use:
brainly.com/question/30861877
#SPJ1
An aqueous KNO3 solution is made using 75.1 g of KNO3 diluted to a total solution volume of 1.95 L .Calculate the molarity of the solution. (assume a density of 1.05 g/mL for the solution)
Calculate the molality of the solution.
Calculate the mass percent of the solution.
Answers
Answer:
- \(M=0.38M\)
- \(\\ \% m=3.67\%\)
Explanation:
Hello,
In this case, since the molar mass of potassium nitrate is 101.1 g/mol, we can compute the molarity as follows:
\(M=\frac{75.1g*\frac{1mol}{101.1g} }{1.95L} \\\\M=0.38M\)
Moreover, as the mass percent is computed as:
\(\% m=\frac{m_{KNO_3}}{m_{solution}} *100\%\)
Thus, by using the given density of the solution, we obtain:
\(\% m=\frac{75.1g}{1.95L*\frac{1000mL}{1L}*\frac{1.05g}{1mL} } *100\%\\\\ \% m=3.67\%\)
Regards.
3. A Wilkinson’s catalyst is widely used in the hydrogenation of alkenes. Show a catalytic cycle, including: i. chemical structure of the catalyst, with complete stereochemistry ii. molecular geometry of catalyst iii. type of reactions involved iv. the appropriate starting material, reagent and solvent v. major and minor end-products vi. all intermediates, for each reaction stated in (iii)
Answers
We can see here that the catalytic cycle for the hydrogenation of alkenes using Wilkinson's catalyst involves several steps.
What are the steps involved?Here's an overview of the catalytic cycle, including the necessary details:
i. Chemical structure of the catalyst:
Wilkinson's catalyst, also known as chloridotris(triphenylphosphine)rhodium(I), has the following chemical structure: [RhCl(PPh3)3]
ii. Molecular geometry of the catalyst:
The Wilkinson's catalyst has a trigonal bipyramidal geometry around the rhodium center. The three triphenylphosphine (PPh3) ligands occupy equatorial positions, while the chloride (Cl) ligand occupies an axial position.
iii. Type of reactions involved:
The catalytic cycle involves several reactions, including:
Oxidative addition: The rhodium center undergoes oxidative addition, reacting with molecular hydrogen (H2) to form a dihydride intermediate.Alkene coordination: The alkene substrate coordinates to the rhodium center, forming a π-complex.Hydrogenation: The coordinated alkene undergoes hydrogenation, resulting in the addition of hydrogen atoms to the double bond and formation of a metal-alkyl intermediate.Reoxidation: The metal-alkyl intermediate reacts with a hydrogen molecule to regenerate the rhodium dihydride species.iv. Starting material, reagent, and solvent:
The starting material is an alkene, and the reagent is Wilkinson's catalyst ([RhCl(PPh3)3]). The reaction is typically carried out in a suitable solvent, such as dichloromethane (CH2Cl2) or tetrahydrofuran (THF).
v. Major and minor end-products:
The major end-product of the hydrogenation reaction is the fully saturated alkane, resulting from the addition of hydrogen across the double bond. The minor end-product may include cis- or trans-configured alkanes if the original alkene substrate possesses geometric isomers.
vi. Intermediates:
The intermediates in the catalytic cycle include:
Rhodium dihydride complex: [RhH2(PPh3)3]Alkene-Rhodium π-complex: [Rh(η2-alkene)(PPh3)3]Metal-alkyl intermediate: [Rh(alkyl)(PPh3)3]These intermediates play a crucial role in facilitating the hydrogenation reaction and enabling the catalytic cycle to proceed.
Learn more about Wilkinson’s catalyst on https://brainly.com/question/31972308
#SPJ1
Sort the five steps of the scientific method.
State problem
Conduct experiment
Interpret data
Draw conclusion
Form hypothesis
Answers
The correct order of the five steps of the scientific method is as follows:
State problem
Form hypothesis
Conduct experiment
Interpret data
Draw conclusion.
The scientific method is a systematic approach used by scientists to investigate and understand the natural world. The five steps of the scientific method, in their logical order, are as follows:
State problem: In this step, the scientist identifies and defines a specific question or problem to be investigated. The problem should be clear and well-defined to guide the rest of the scientific process.
Form hypothesis: A hypothesis is a proposed explanation or prediction for the problem stated in step one. It is an educated guess that can be tested through experiments and observations. The hypothesis should be based on prior knowledge and observations.
Conduct experiment: In this step, the scientist designs and performs experiments to test the hypothesis. The experiment is carefully planned and executed, and data is collected through observations and measurements.
Interpret data: Once the experiment is completed, the scientist analyzes the collected data. This involves organizing, graphing, and statistically analyzing the data to identify patterns and trends.
Draw conclusion: Based on the interpretation of the data, the scientist draws conclusions about whether the hypothesis is supported or not. The conclusions should be objective and supported by evidence obtained from the experiment.
It's important to note that while these steps are presented in a linear order, the scientific process is often iterative, with scientists revisiting and refining hypotheses, conducting further experiments, and building upon existing knowledge.
For more questions on Conduct experiment
https://brainly.com/question/30994979
#SPJ8
4.Using their general formula, CnH2n-2 derive the molecular formula of the alkynes when n = 5, 6, 7, 8)
5.Write the condensed structural formula of the molecules in #4.
Answers
C₅H₆ is the first pentyne, C₆H₁₂ is the first hexyne, C₇H₁₀ is the first heptyne,
C₈H₁₂ is the last pentyne (this is 1-octyne). pentyne: 1-Butyne, hexyne: 3-methylpentane, heptyne: 3,3-dimethylbut-1- ene, and octyne: 5-(2-methylpropyl)-decane
Alkynes have the generic formula :Alkynes are hydrocarbons with at least one triple bond between two carbon atoms. Alkynes have the general formula. Alkyne must contain at least two carbon atoms, much like alkene.
What does the general formula represent?The -ane ending in the name indicates that the substance is an alkane. Methane, ethane, propane, butane, pentane, hexane, heptane, and octane are the names of the alkanes. The general formula for alkanes.
To know more about heptyne :
brainly.com/question/24229597
#SPJ1
The following balanced equation represents what type of reaction?
2 H2O → 2 H2 + O2
Select one:
a.
double displacement
b.
single displacement
c.
synthesis
d.
decomposition
Answers
Answer:
D.) decomposition
Explanation:
A.) is incorrect. In double-displacement reactions, the cation of one compound is swapped with the cation of another.
These reactions have the general structure: AB + CD -----> AD + BC.
B.) is incorrect. In single-displacement reactions, the an element within a compound is swapped with another element.
These reactions have the general structure: AB + C -----> AC + B.
C.) is incorrect. In synthesis reactions, two or more elements/compounds are combined to create a new and larger compound/molecule.
These reactions have the general structure: A + B -----> AB.
D.) is correct. In decomposition reactions, a compound/molecule is broken down into smaller elements/compounds. In the given reaction, water (H₂O) separates into its respective elements, hydrogen (H₂) and oxygen (O₂).
These reactions have the general structure: AB -----> A + B