Determine the mass percent K2C2O4 in a sample Unknown X, given the following information. A sample of 1.25 g Unknown X was dissolved in 25 mL DI water and 25 mL 3 M H2SO4. The sample was titrated with 0.0421 M KMnO4 solution and it took 33.52 mL to reach the endpoint.
Answers
The mass percent K2C2O4 in a sample Unknown X is determined below The molar mass of K2C2O4 is 245.26 g/mol. The balanced equation for the reaction is:K2C2O4 + KMnO4 + H2SO4 → K2SO4 + MnSO4 + CO2 +
H2OFrom the are equation stoichiometry between KMnO4 and K2C2O4 is The reaction equation is used to calculate the number of moles of K2C2O4 in Unknown X .From the balanced equation above,1 mol KMnO4 reacts with 1 mol K2C2O4 moles of K2C2O4 in Unknown X = moles of KMnO4 used Since the concentration of KMnO4 used is given as 0.0421 M (Molar concentration or molarity),then moles of KMnO4 = (0.0421 mol/dm³)(33.52 mL)(1 dm³/1000 mL) = 0.001410 dm³The volume of the solution of Unknown X is given as 25 mL (milliliters), therefore its concentration can be calculated as follows Concentration of K2C2O4 = moles of K2C2O4 / volume of solution of Unknown X in liters= (0.001410 mol) / (25 mL/1000) L= 0.0564 mol/L= 5.64 g/LThis means that in 1 L of solution of Unknown X,
there are 5.64 g of K2C2O4.In 25 mL of solution of Unknown X, there are:5.64 g/L × 25 mL / 1000 mL = 0.141 g of K2C2O4 The mass percent K2C2O4 in a sample Unknown X can be determined by taking the mass of K2C2O4 present in the sample as a fraction of the total mass of the sample and then multiplying by 100%. Concentration of K2C2O4 = 5.64 g/L The volume of the solution of Unknown X is given as 25 mL (milliliters), therefore its concentration can be calculated as follows Concentration of K2C2O4 = (0.0564 mol/L)×(2 mol K2C2O4/1 mol KMnO4)×(245.26 g K2C2O4/1 mol K2C2O4)= 27.72 g/L Mass of K2C2O4 in Unknown X = (27.72 g/L)×(25 mL/1000 mL)= 0.693 gMass percent K2C2O4 in Unknown X = (Mass of K2C2O4 in Unknown X / Mass of Unknown X) × 100%= (0.693 g / 1.25 g) × 100%= 55.44%Therefore, the mass percent K2C2O4 in a sample Unknown X is 55.44%.
To know more about equation Visit;
https://brainly.com/question/3049557
#SPJ11
Related Questions
Why is glass useful for making eyeglasses
Answers
Answer:
because it's the glass that goes on eyeglasses
Answer:
Because it is transparent
What type of bond attracts one water molecule to another?.
Answers
Answer:
Hydrogen Bonds
Explanation:
In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules. The attraction between individual water molecules creates a bond known as a hydrogen bond.
Hi I need help on this question quick please!!

Answers
4. What gives blood its red color?
a. hemoglobin
O
b. red blood cells
O
c. platelets
d. plasma
Answers
Answer:
the best answer would be A
Explanation:
cause it contains a red colored compound.
does the grain size-number (g of equation 4.17) increase or decrease with decreasing grain size?
Answers
As the grain size decreases, the grain size-number (g) in the Hall-Petch equation increases.
How to describes the relationship between the yield strength of a metal and its grain size?Equation 4.17, commonly known as the Hall-Petch equation, relates the yield strength of a metal to its grain size:
\(σy = σ0 + Kd^(-1/2)\)
where σy is the yield strength, σ0 is the frictional stress, K is the Hall-Petch constant, and d is the average grain size.
According to the Hall-Petch equation, the yield strength of a metal increases with decreasing grain size. This means that the value of the Hall-Petch constant K is positive, indicating that the yield strength increases as the grain size decreases.
In other words, as the grain size decreases, the grain size-number (g) in the Hall-Petch equation increases.
Learn more about Hall-Petch equation
brainly.com/question/12975121
#SPJ11
What Element am I?
I have 6 Valence Electrons.
I am in the third row.
My atomic mass is less than Selenium , but it is more than Oxygen. I need the answer ASAP
Answers
Answer:
The correct answer is - sulfur.
Explanation:
In the periodic table, there are 18 groups and 7 rows or periods arranged according to their atomic number or electronic configuration. In the question, it is mentioned that the desired element atomic mass is less than the atomic mass of the selenium which is 78.96, and more than oxygen which is 15.99 with 6 electron valence and present in the third row.
As it has 6 valency of electron it must be in the 16 group of the table that comprises the 6 valency and as it is located in the 3rd row it must be sulfur that also has an atomic mass between selenium and oxygen.
Which best describes the law of conservation of mass? The coefficients in front of the chemicals in the reactants should be based on the physical state of the products. Products in the form of gases are not considered a part of the total mass change from reactants to products. When reactants contain both a solid and a liquid, the solid counts toward the overall mass and the liquid does not. The mass of the reactants and products is equal and is not dependent on the physical state of the substances.
Answers
Answer:
D) The mass of the reactants and products is equal and is not dependent on the physical state of the substances.
Explanation:
I took the quiz on Edgenuity
The best description of law of conservation of mass is the mass of the reactants and products is equal and is not dependent on the physical state of the substances. Thus, option D is correct.
What is law of conservation of mass?
Law of conservation stats that the any closed in which mass or energy transfer is takes placed the sum of energy and mass before or after transfer is remain constant.The mass of the reactants and products is equal and is not dependent on the physical state of the substances.
It means that in terms of physics if any closed or open system if mass or energy transfer is takes place from the system the total energy i.e in system or surrounding the total energy will always constant. Law of conservation stats that the any closed in which mass or energy transfer is takes placed the sum of energy and mass before or after transfer is remain constant.
Therefore,the best description of law of conservation of mass is the mass of the reactants and products is equal and is not dependent on the physical state of the substances. Thus, option D is correct.
Learn more about law of conservation of mass here:
https://brainly.com/question/13383562
#SPJ6
A compound with the empirical formula CH2 was found to have a molar mass of approximately 84g. What is the molecular formula of the compound?
Answers
Answer:
C6H12
Explanation:
Molar mass of C =12.0 H=1.0
12x + 2(1x) = 84
x = 6
so it should be C6H12
The molecular formula of the compound will be C₇H₁₄.
What is the empirical formula?The empirical formula of the compound gives the simplest ratio of the number of different elements present in a compound. The molecular formula of the compound provides the actual number of each element present in a compound. This formula is generally used and can be determined as a multiple of the empirical formula of a compound.
The empirical formula offers the ratio of the number of each atom in a given compound. The composition of a compound is directly determined by its empirical formula.
Given, the empirical formula of the compound is CH₂.
The empirical formula mass of the compound = 12 + 2 = 14 g
The molar mass of the compound = 84 g
The multiple will be = 84/12 = 7
The molecular formula of the compound = (CH₂)₇ = C₇H₁₄
Therefore, the molecular formula of the compound is C₇H₁₄.
Learn more about the empirical formula, here:
brainly.com/question/14044066
#SPJ2
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
How many molecules are there in 0.5 moles of C2H6?
Answers
Answer:
3
Explanation:
points
Sodium and chlorine react together to form sodium chloride. Identify the reactant(s) and product(s?
Answers
Sodium and chlorine are the two reactants and sodium chloride is the product.
A very reactive metal called sodium interacts with chlorine gas to form sodium chloride, an inert salt. Chlorine gas is reduced to chloride anions whereas sodium is oxidized to sodium cation (Na+) (Cl-).One mole of sodium and two moles of chlorine gas combine to form two moles of sodium chloride.We are aware that chlorine is a very reactive non-metal and that sodium is a very active metal. Typically, non-metals like halogens tend to receive electrons while metals prefer to expel electrons.Chlorine accepts the electron to create the anion Cl-, and sodium rapidly loses its final shell electron to produce the cation Na+.Sodium, a solid reactant, interacts with greenish-yellow gaseous chlorine to form white crystalline sodium chloride.
Therefore, sodium and chlorine are reactants whereas sodium chloride is the product.
Learn more about chemical reactions:
https://brainly.com/question/11231920
#SPJ9
Please Help
1. Convert 1.65 moles of magnesium chloride to grams
2. how many moles are in 100 grams of methane (CH4)
Answers
Answer:
157 grams magnesium chloride
Explanation:
1. We must first find the molar mass (g/mole) of magnesium chloride. The molecular formula is MgCl2.
Add the elemental atromic weights for one molecule of the material:
1 Mg = 24.3
2 Cl = 2*(35.45) = 70.9
Total = 95.2 grams/mole
We can use this as a conversion factor by multiplying it times the moles we are asked to convert: (1.65 moles MgCl2)*(95.2 grams/mole MgCl2).
The moles cancel, leaving us with 157 grams of magnesium chloride.
In which type of medium would light waves travel in a straight line?
O a medium that is uneven
O a medium that is uniform
O a medium composed of different densities
O a medium that is varied
Answers
Light will travel in a straight line in a medium that is uniform. Option 2.
Medium of light travelLight waves would travel in a straight line through a homogeneous or uniform medium. Such media are media that have a constant refractive index throughout their volume.
Examples of such mediums include air, vacuum, and certain types of glass or plastic. When light enters a medium with a varying refractive index, such as water or a prism, it can be refracted or bent from its original path.
More on the medium of light traveling can be found here: https://brainly.com/question/23472237
#SPJ1
(3) Describe what you would see if you added 1. a piece of zinc metal to a test tube that contains 6-molar hydrochloric acid; 2. a piece of copper metal to another test tube that contains 6-molar hydrochloric acid. (c) Write balanced equations for any reactions that occur. (c) Explain how you could use the table of standard reduction potentials to predict the observed results.
Answers
Zinc reacts vigorously with hydrochloric acid, producing zinc chloride and hydrogen gas, while copper does not react significantly due to its lower reactivity.
If you add a piece of zinc metal to a test tube containing 6-molar hydrochloric acid, you would observe a vigorous reaction. Bubbles of gas would be released, and the solution would become cloudy. The zinc metal would dissolve in the hydrochloric acid, producing zinc chloride and hydrogen gas. The balanced equation for this reaction is:
\(Zn(s) + 2HCl(aq) \rightarrow ZnCl_2(aq) + H2(g)\)
On the other hand, if you add a piece of copper metal to another test tube containing 6-molar hydrochloric acid, you would not observe a significant reaction. Copper is less reactive than zinc, so it does not readily react with hydrochloric acid. The balanced equation for this case would be:
\(Cu(s) + 2HCl(aq) \rightarrow no reaction\)
To predict these observations using the table of standard reduction potentials, you would compare the reduction potentials of zinc and copper.
Zinc has a more negative reduction potential compared to hydrogen, indicating that it is more likely to undergo oxidation (lose electrons) and react with the acid to produce hydrogen gas. Copper, on the other hand, has a higher reduction potential than hydrogen, indicating that it is less likely to undergo oxidation and react with the acid.
Therefore, the observations align with the predicted reactivity based on the standard reduction potentials.
To learn more about zinc chloride from the given link
https://brainly.com/question/32106372
#SPJ4
16. Keesha performed a chemical reaction, and the products looked quite
different from the reactants. She knew the amount of matter had not
changed due to the law of conservation of mass. According to the law of
conservation of mass, what happens in a chemical reaction? *

Answers
Answer:
it's the third one
Explanation:
matter can't change, but mass can differ
In a balanced chemical reaction, the number of atoms of each element in the product(s) always equals the
A. molar mass of the reactants
B. proportional masses of the reactants
C. the number of atoms of each element in the reatants
D. the number of elements in the reactants
Answers

what are three properties of non-metals
Answers
Answer:
Brittle, Poor Conductors, Non-Malleable
Explanation:
Non-metals are the opposite of metals; they have opposite properties.
Metals are ductile, malleable, conductive, and shiny, while nonmetals are the opposite.
If there are 0.090 moles of sugar (C₁₂H₂₂O₁₁) in the average can of soda, how many sugar molecules would this be
Answers
Given :
There are 0.090 moles of sugar (C₁₂H₂₂O₁₁) in the average can of soda.
To Find :
How many sugar molecules would this be ?
Solution :
We know, in 1 mole of any compound their are \(6.022\times 10^{23}\) molecules of that compound.
So, number of molecules in 0.090 moles of compound is :
\(n = 0.090 \times 6.022 \times 10^{23}\\\\n = 5.42\times 10^{22}\)
Therefore, number of molecules in 0.090 moles of compound is \(5.42 \times 10^{22}\) .
plz help it is due rn, help ASAP

Answers
Answer:
A
Explanation:
3. This experiment required that you change the solutions between electrochemical cells even if they used the same reagents. If you had not done this the potentials observed might become less accurate. Why was this the case
Answers
The reason why changing the solutions between electrochemical cells is necessary, even if they use the same reagents, is because there may be differences in the concentrations of the reagents or impurities in the solutions that can affect the measured potential.
In the experiment where you change the solutions between electrochemical cells even if they used the same reagents, the potentials observed might become less accurate if you had not done this. This is because, over time, the concentrations of the solutions and the electrode surfaces can change due to reactions occurring at the electrodes. This can lead to alterations in the potentials and a decrease in accuracy. Therefore, it is important to change the solutions to maintain consistency and achieve accurate results.
Learn more about electrochemical cells: https://brainly.com/question/31149864
#SPJ11
what might happen if a student used a pen to mark the baseline on the chromatography paper?
Answers
If a student used a pen to mark the baseline on the chromatography paper, it could affect the results of the experiment.
Chromatography is a laboratory technique used to separate and identify the components of a mixture based on their properties, such as solubility and molecular weight. In paper chromatography, a small amount of the mixture to be analyzed is placed on a strip of filter paper, and the paper is then placed in a solvent.
As the solvent moves up the paper, it carries the different components of the mixture with it, separating them based on their properties.
The baseline is a line drawn near the bottom of the paper that marks the starting point of the experiment. If a pen is used to mark the baseline, it can interfere with the separation of the components of the mixture by interacting with the solvent or the components themselves. This can result in inaccurate or unreliable results, which can impact the conclusions drawn from the experiment. Therefore, it's important to use a pencil or other non-reactive material to mark the baseline on chromatography paper.
To know more about chromatography paper,
https://brainly.com/question/31857177
#SPJ11
calculate the ????∘ for the following equation. use these standard potentials.clo−4(aq) 6h3o (aq) 6br−(aq)⟶3br2(aq) clo−(aq) 9h2o(l)
Answers
The question is asking for the standard reduction potential (E°) of the given equation. To calculate E°, we need to use the standard reduction potentials of the species involved. The reduction potential for the half-reaction is determined by subtracting the reduction potential of the reactant from the reduction potential of the product.
Given the equation:
ClO₄⁻(aq) + 6H₃O⁺(aq) + 6Br⁻(aq) ⟶ 3Br₂(aq) + ClO⁻(aq) + 9H₂O(l)
We can break it down into two half-reactions:
1. ClO₄⁻(aq) + 8H⁺(aq) + 6e⁻ ⟶ ClO⁻(aq) + 4H₂O(l)
2. 6Br⁻(aq) ⟶ 3Br₂(aq) + 6e⁻
Now, we can look up the standard reduction potentials for each half-reaction. Subtracting the reduction potential of the reactant from the reduction potential of the product for each half-reaction gives us the reduction potentials. Finally, sum the reduction potentials of both half-reactions to get the overall reduction potential of the equation.
To know more about reduction potential visit:-
https://brainly.com/question/33594918
#SPJ11
Express in scientific notation. Choose the answer with the proper number of significant figures.
(3x10^4) (4x10^23)
A: 10x10^27
B: 1x10^28
C: 12^27
D: 12x10^27
Answers
This is because the 3 is distributed to the 4 to get 12. The 10^4 is distributed to the 10^23 and because of the exponent laws, the exponents are added to get 27 and the 10's (being the base) is left alone.
A 250. cm3 sample of neon is collected at standard temperature and pressure. Assuming the volume remains constant, what would be the pressure of the neon at 44.0°C?
a) 118 b)87.2 c) 0.00 d)290
Answers
Answer:
The answer is 118 kPa.
Explanation:
What would happen if sodium and potassium are placed in hot water?
Answers
All the alkali metals react vigorously with cold water. In each reaction, hydrogen gas is given off and the metal hydroxide is produced. The speed and violence of the reaction increases as you go down the group. This shows that the reactivity of the alkali metals increases as you go down Group 1.
Sodium
When sodium is added to water, the sodium melts to form a ball that moves around on the surface. It fizzes rapidly, and the hydrogen produced may burn with an orange flame before the sodium disappears.
Potassium
When potassium is added to water, the metal melts and floats. It moves around very quickly on the surface of the water. The hydrogen ignites instantly. The metal is also set on fire, with sparks and a lilac flame. There is sometimes a small explosion at the end of the reaction.
which gas is not an example of a naturally occurring greenhouse gas?
Answers
nitrogen and chloro fluro carbon gas is not an example of a naturally occurring greenhouse gas
Water vapour, carbon dioxide, methane, nitrous oxide, and ozone are examples of naturally occurring greenhouse gases. Hydro-fluorocarbons (HFCs), perfluorocarbons (PFCs), and sulphur hexafluoride (SF6) are examples of artificial greenhouse gases that are produced via a number of industrial operations. Carbon dioxide, methane, ozone, nitrous oxide, chlorofluorocarbons, and water vapour are some examples of greenhouse gases. The gas that is not a greenhouse gas is therefore nitrogen. The different greenhouse gases include nitrous oxide, water vapour, ozone, chlorofluorocarbons, methane, and chlorofluorocarbons. Infrared light cannot pass through oxygen or nitrogen, hence they are not considered greenhouse gases. These molecules are invisible because stretching one of them has no effect on the electric field. These symmetric molecules are composed of two identical atoms whose electric fields simply cancel one another out.
which gas is not an example of a naturally occurring greenhouse gas?
1. nitrogen
2.cholrofluoro carbons
3. oxygen
4. carbondioxide
Learn more about nitrogen here:
https://brainly.com/question/16711904
#SPJ4
The products obtained by the acid-catalyzed hydration of 1-methylcyclohexene and methylenecyclohexene are identical
Answers
The statement “The products obtained by the acid-catalyzed hydration of 1-methylcyclohexene and methylenecyclohexene are identical” is not true.
Acid-catalyzed hydration is a chemical reaction that transforms an alkene to an alcohol by adding water.
For instance, in the following reaction, 2-methylpropene reacts with water, with the aid of sulfuric acid, to give the alcohol tert-butyl alcohol.
In general, acid-catalyzed hydration of an alkene takes place via a mechanism known as a Markovnikov addition. When an unsymmetrical alkene is hydrated using H2SO4 and H2O, a mixture of products is usually produced. This is because of the differing stabilities of the carbocation intermediates formed during the reaction. This is referred to as carbocation rearrangement.
A simple example of carbocation rearrangement can be seen in the hydration of 1-methylcyclohexene. It produces two isomeric products: 1-methylcyclohexanol and 3-methylcyclohexanol.
Methylcyclohexene undergoes acid-catalyzed hydration to form a mixture of 1-methylcyclohexanol and 3-methylcyclohexanol. Methylenecyclohexene, on the other hand, yields only cyclohexanol during hydration.
Thus, the given statement is false.
To learn more about Markovnikov addition :
https://brainly.com/question/21496002
#SPJ11
PLEASE HELP ME!!!
THE CHEM QUESTION IS...
Calculate the amounts of heat required for 25.0 g of water to change from 10°C to 30°C
Answers
Answer:
1045
Answer: 1045 J of energy was released on cooling the down the water from 20 °C to 10 °C.
Answer:
..............=2100
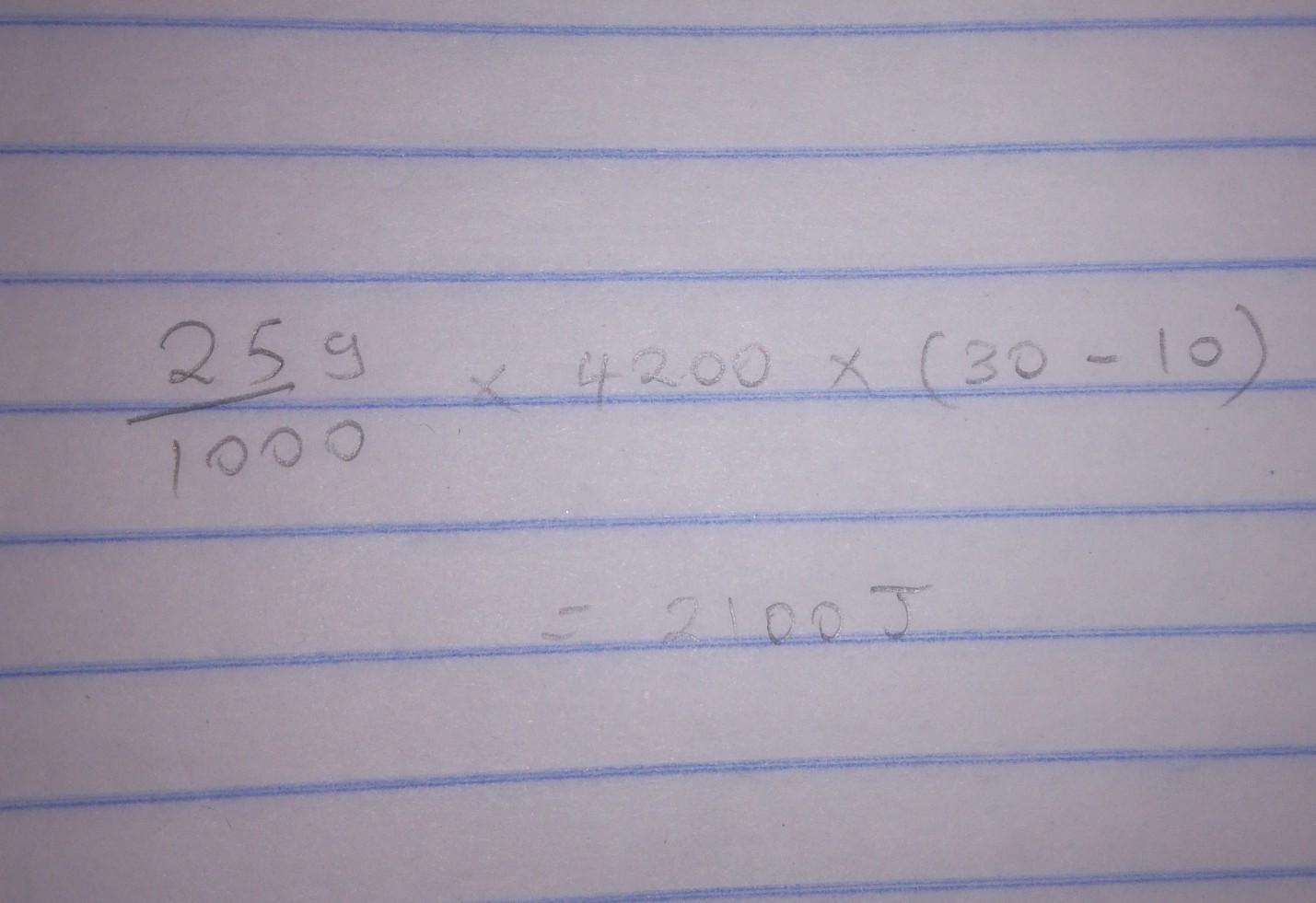
if you start with 10.38 g of crude sulfanilamide and recrystallize it from ethanol to recover 5.74 g of pure sulfanilamide, what is the percent recovery? provide your answer as a whole number.
Answers
The percent recovery of pure sulfanilamide is approximately 55.2%. Percent recovery is a measure of the efficiency of a purification process, such as recrystallization.
To calculate the percent recovery of pure sulfanilamide, we need to compare the mass of the recovered pure sulfanilamide with the initial mass of crude sulfanilamide and express it as a percentage.
The percent recovery can be calculated using the formula:
Percent Recovery = (Mass of Recovered Sulfanilamide / Initial Mass of Crude Sulfanilamide) × 100
Given:
Initial mass of crude sulfanilamide = 10.38 g
Mass of recovered pure sulfanilamide = 5.74 g
Substituting these values into the formula, we get:
Percent Recovery = (5.74 g / 10.38 g) × 100
Percent Recovery = 0.552 × 100
Percent Recovery ≈ 55.2%
It represents the proportion of the desired substance that is successfully recovered from the crude material. In this case, 55.2% of the initial crude sulfanilamide was successfully recovered as pure sulfanilamide through the recrystallization process.
It's important to note that the percent recovery can be influenced by various factors, such as the efficiency of the purification technique, loss during filtration or transfer, and the purity of the starting material. In practice, a higher percent recovery is desirable, indicating a more efficient purification process.
Learn more about sulfanilamide at: brainly.com/question/32096446
#SPJ11
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11