Answers
Related Questions
A 1.0 x10 - 4 M solution has a pH of 10.00. The solute is a _____.Select one:a. weak acidb. weak basec. strong acidd. strong basei put d but answer was wrong can you explain how to figure this out
Answers
1) List the known and unknown quantities.
Sample: unknown.
Concentration: 1.0*10^(-4).
pH: 10.00.
Characteristic: unknown.
2) pH scale.
Weak acid: 4 - 6
Weak base: 8 - 10
Strong acid: 1 - 3
Strong base: 11 - 14
According to the more specific pH scale above, 10.00 corresponds to a weak base.
Option B.


If potassium reacts so dangerously with water, and people are mostly made of water, why can we eat bananas without blowing up?
Answers
Answer:
A banana, like most other lifeforms, is about 70% water, and all the potassium it contains already exists in the form of K+ ions dissolved in water. Which is why bananas do not explode in water, or spontaneously combust.
Using the Cell Size and Scale Interactive, tell me which of the following is correct. They are in order of size, left to right, from smallest to largest:
tRNA > influenza virus > E. coli bacterium
Hepatitis virus > hemoglobin > phospholipid
Ribosome > amoeba proteus > carbon atom
Answers
Using the cell size and scale Interactive, the correct order of size, left to right, from smallest to largest is tRNA > influenza virus > E. coli bacterium Hepatitis virus > hemoglobin > phospholipid. The correct option is a.
What is t-RNA?t-RNA is transfer RNA. It helps in transferring information from RNA to DNA or DNA to RNA.
t-RNA is present in viruses, so it will be smaller than the virus because they are present inside the virus. Phospholipids are chains of lipids. They are macromolecules.
Therefore, the correct option is a. tRNA > influenza virus > E. coli bacterium Hepatitis virus > hemoglobin > phospholipid.
To learn more about Cell Size and Scale Interactive, refer to the link:
https://brainly.com/question/917170
#SPJ1
What type of climate conditions are needed to form coal deposits.
Answers
Answer:
Subbituminous coal can form at temperatures as low as 35 to 80 °C (95 to 176 °F) while anthracite requires a temperature of at least 180 to 245 °C (356 to 473 °F).
Sub-types: Cannel coal
Child material class: Lignite
Explanation:
N2(g)+3H2(g)->2NH3(g), ΔH=-92.40kJ 1. How many grams of H2 are needed to involve 150.9kJ of heat? 2. How many moles of NH3 were produced in the process?
Answers
1. To solve for the grams of H2 needed, we need to use the given ΔH value to calculate the amount of moles of N2 that reacted. From the balanced chemical equation, we know that for every 3 moles of H2 that reacts, 1 mole of N2 reacts. Therefore, we can use the mole ratio to convert the moles of N2 to moles of H2 and then use the molar mass of H2 to convert to grams.
First, we need to calculate the moles of N2 that reacted to produce 150.9kJ of heat:
ΔH = -92.40 kJ/mol N2
150.9 kJ = (1 mol N2 / -92.40 kJ) x (-150.9 kJ)
mol N2 = 1.63 mol
Using the mole ratio from the balanced chemical equation:
1 mol N2 : 3 mol H2
We can calculate the moles of H2 needed:3 mol H2 = 1 mol N2
3 mol H2 = 1.63 mol N2
mol H2 = 0.543 mol
Finally, we can convert moles of H2 to grams:
mol H2 = 0.543 mol
molar mass of H2 = 2.02 g/mol
grams of H2 = (0.543 mol) x (2.02 g/mol)
grams of H2 = 1.10 g
Therefore, 1.10 grams of H2 are needed to involve 150.9kJ of heat.
2. To solve for the moles of NH3 produced, we can use the same mole ratio from the balanced chemical equation:
1 mol N2 : 2 mol NH3
From the moles of N2 that reacted calculated in part 1, we can calculate the moles of NH3 produced:
1 mol N2 = 2 mol NH3
1 mol N2 = 1.63 mol N2
mol NH3 = (2 mol NH3 / 1 mol N2) x (1.63 mol N2)
mol NH3 = 3.26 mol
Therefore, 3.26 moles of NH3 were produced in the process.
For more questions on: moles
https://brainly.com/question/15356425
#SPJ11
What is the empirical formula for a compound that has 57.5%
Na, 40.0% 0, and 2.5% H?
a
Naz(OH)4
b
Na(OH)2
С
Na(OH)
d
Naz(OH)
Answers
Answer:
c
Explanation:
use %composition given to calculate emperical 4mular
The empirical formula of the compound having 57.5% Na, 40.0% O, and 2.5% H is NaOH (Option C)
Data obtained from the questionNa = 57.5%O = 40%H = 2.5%Empirical formula =?How to determine the empirical formulaDivide by their molar mass
Na = 57.5 / 23 = 2.5
O = 40 / 16 = 2.5
H = 2.5 / 1 = 2.6
Divide by the smallest
Na = 2.5 / 2.5 = 1
O = 2.5 / 2.5 = 1
H = 2.5 / 2.5 = 1
Thus, the empirical formula of the compound is NaOH
Learn more about empirical formula:
https://brainly.com/question/24297883
#SPJ2
How Many atoms of each element are Ca(NO3)2)
Answers
Help with science ASAP!! Will give brainliest!! :)

Answers
B
Explanation:
20.0g+25.0g+30.0g=75.0g
75.0g/3=25.0g
If [H3O^ + ]=1.7*10^ -8 M what is the pOH of the solution?
Answers
Answer: 6.23
Explanation:
1) solve for pH
pH=-log (H3O+) = - log 1.7 X 10^-8 =7.77
2) now do 14-pH = 14 -7.77=6.23
why blood is separated into different parts
Answers
Answer:
Blood fractionation is the process of fractionating whole blood, or separating it into its component parts. This is typically done by centrifuging the blood. The resulting components are: a clear solution of blood plasma in the upper phase (which can be separated into its own fractions, see Blood plasma fractionation),
Answer: Centrifugal force is used to separate the components of blood – red blood cells, platelets and plasma – from each other. ... The red blood cells precipitate to the bottom of the bag, with the platelets above them, then the white blood cells and the plasma at the very top. Also because Each part of the blood has a different function. Separating the blood into parts lets patients get only the specific part or parts of the blood that they need. So a whole blood donation can be used for several patients.
Hope this helps have a awesome day/nigh❤️✨t
Explanation:

What part of the underside of a gecko's feet
allows it to stick to surfaces?
O cilia
O heat
O pillars
O webbing
Answers
Answer:
it was pillars
Explanation:
i guessed and it was right LOL
Answer:
O pillars
Explanation:
Right on EDGE 2021
If 255 J of heat is applied to 20.0 g of
gold, what temperature change will
occur if the specific heat of gold is 0.129 j/g C
Answers
Answer:
ΔT = 98.84 °C
Explanation:
Given data:
Heat absorbed = 255 J
Mass of gold = 20.0 g
Specific heat capacity of gold = 0.129 J/g.°C
Temperature change = ?
Solution:
Formula:
Q = m.c. ΔT
Q = amount of heat absorbed or released
m = mass of given substance
c = specific heat capacity of substance
ΔT = change in temperature
by putting values,
255 J = 20.0 g × 0.129 J/g.°C × ΔT
255 J = 2.58 J / °C × ΔT
ΔT = 255 J / 2.58 J / °C
ΔT = 98.84 °C
A single atom with a unique number of protons
Answers
Answer:
One atom of that element like helium neon and other Noble gases
Explanation:
Answer:
Please make sure to re-write this on your own so your teacher doesn't think you cheating!Explanation:
The number of protons in the nucleus of an atom determines an element's atomic number. In other words, each element has a unique number that identifies how many protons are in one atom of that element. An example is all hydrogen atoms, and only hydrogen atoms, contain one proton and have an atomic number of 1.
The equation for the synthesis of ammonia is N2 + 3 H2 -> 2 NH3. How many moles of H2 I needed to produce 6 mol of NH3? A. 4 B. 6 C. 8 D. 9

Answers
The equation for the synthesis of ammonia is:
N₂ + 3 H₂ ---> 2 NH₃
We have to find the number of moles of H₂ needed to produce 6 mol of NH₃. To do that we will use the equation that we were given.
We can read the equation of the reaction as a recipe. When 1 mol of N₂ reacts with 3 moles of H₂, 2 moles of NH₃ are produced. So the relationship between H₂ and NH₃ is that 3 moles of H₂ are needed to produce 2 moles of NH₃. We wiill use that relationship to solve the problem.
6 moles of NH₃ * 3 moles of H₂/(2 moles of NH₃) = 9 moles of H₂
Answer: D) 9 moles of H₂ are needed.
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
How many molecules are there in 2.3 grams of NH4SO2?
Answers
Answer:
Hence, there are approximately $1.686\times {{10}^{22}}$ molecules in 2.3 grams of $N{{H}_{4}}S{{O}_{2}}$.
Use your periodic table to answer the following questions. Pay attention to the spelling of the element names and make sure to write the chemical symbols correctly. 1. What metal is found in the group 1 and period 4? __________________ 2. What metalloid is found in the group 16 and period 5? ______________ 3. What nonmetal is found in the group 17 and period 2? ______________ 4. In what group will you find copper? ____________________ 5. In what group will you find krypton? ____________________ 6. In what group will you find mercury? _______________________ 7. What is the symbol for chlorine? ___________________ 8. What is the symbol for lead? _____________________ 9. What is the symbol for tungsten? ______________________ 10. What is the symbol for antimony? _______________________ 11. What is the symbol for sodium? ______________________ 12. In what period will you find gold? __________________________ 13. In what period will you find silver? _________________________ 14. In what period will you find oxygen? _________________________ 15. In what groups will you find metalloids? ______ ______ _____ _____ 16. What element has the symbol H? _______________________ 17. What element has the symbol He? _______________________ 18. What element has the symbol Ca? ________________________ 19. What element has the symbol Co? ________________________ 20. What element has the symbol C?
Answers
Explanation:
1. K (Potassium)
2. Te (Tellurium)
3. F (Flourine)
4. group 11, period 4
5. group 18, period 4
6. group 12, period 6
7. Cl
8. Pb
9. W
10. Sb
11. Na
12. period 6, group 11
13. period 5, group 11
14. period 2, group 16
15. Groups 13–16 of the periodic table contain one or more metalloids
16. Hydrogen
17. Helium
18. Calcium
19. Cobalt
20. Carbon
1. K (Potassium)metal is found in the group 1 and period 4.
2. Te (Tellurium) metalloid is found in the group 16 and period 5.
3. F (Flourine)nonmetal is found in the group 17 and period 2.
4. Group 11, period 4 group will you find copper.
5. Group 18, period 4 group will you find krypton.
6. Group 12, period 6 group will you find mercury.
7. Cl is the symbol for chlorine.
8. Pb the symbol for lead.
9. W the symbol for tungsten.
10. Sb is the symbol for antimony.
11. Na is the symbol for sodium.
12. Period 6, group 11 period will you find gold.
13. Period 5, group 11 period will you find silver.
14. Period 2, group 16 of the periodic table contain one or more metalloids.
15. Groups 13–16 groups will you find metalloids.
16. Hydrogen element has the symbol H.
17. Helium element has the symbol He.
18. Calcium element has the symbol Ca.
19. Cobalt element has the symbol Co.
20. Carbon element has the symbol C.
What is mixture and compound?In chemistry mixture is a combination of two or more different chemical elements which are not chemically bond. A mixture is the physical combination of two or more elements in which their individual identities are retained and it also mixed by solutions, suspension and colloids.
In chemistry compound is combination of two or more different elements which are chemically bond. So compound is held by chemical bond. A compound can't be separate by physical separation and after the mixture you can't identified that it is compound or not.
Therefore, 1. K (Potassium)metal is found in the group 1 and period 4.
2. Te (Tellurium) metalloid is found in the group 16 and period 5.
3. F (Flourine)nonmetal is found in the group 17 and period 2.
4. Group 11, period 4 group will you find copper.
5. Group 18, period 4 group will you find krypton.
6. Group 12, period 6 group will you find mercury.
7. Cl is the symbol for chlorine.
8. Pb the symbol for lead.
9. W the symbol for tungsten.
10. Sb is the symbol for antimony.
11. Na is the symbol for sodium.
12. Period 6, group 11 period will you find gold.
13. Period 5, group 11 period will you find silver.
14. Period 2, group 16 of the periodic table contain one or more metalloids.
15. Groups 13–16 groups will you find metalloids.
16. Hydrogen element has the symbol H.
17. Helium element has the symbol He.
18. Calcium element has the symbol Ca.
19. Cobalt element has the symbol Co.
20. Carbon element has the symbol C.
Learn more about compound and mixture here:
https://brainly.com/question/5169457
#SPJ2
Choose the answer that lists the CO₂ equivalents in order for methane,
nitrous oxide, and CFC-12.
HUMAN-RELEASED GREENHOUSE GASES
Carbon Dioxide
Methane
Nitrous Oxide
CFC-12
Current
Concentration
1860 ppb 86-12
332 ppb 268 -114
500 ppt 11,000 100
A. 0.01, 0.86, 2.68
B. 0.01, 0.86, 110
C. 0.86, 2.68, 110
D. 0.01, 2.68, 110
GWP for
20 years
410 ppm 1 Multiple
Atmospheric
Lifetime (years)

Answers
The correct answer is C: 0.86, 2.68, 110. The Global Warming Potential (GWP) is a measure of how much a given mass of a greenhouse gas contributes to global warming, relative to the same mass of carbon dioxide (CO₂).
The GWP takes into account the radiative forcing of each greenhouse gas and its atmospheric lifetime.
For a time horizon of 20 years, the GWP values are:
Methane: 86
Nitrous oxide: 268
CFC-12: 11,000
These values indicate that, over a 20-year period, methane is 86 times more potent as a greenhouse gas than CO₂, nitrous oxide is 268 times more potent, and CFC-12 is 11,000 times more potent.
To convert the GWP values to CO₂ equivalents, we multiply each value by the GWP of CO₂ (which is defined as 1). Therefore, the CO₂ equivalents for methane, nitrous oxide, and CFC-12 are:
Methane: 0.86 CO₂ equivalents
Nitrous oxide: 2.68 CO₂ equivalents
CFC-12: 110 CO₂ equivalents
Therefore, the correct answer is C: 0.86, 2.68, 110, as it lists the CO₂ equivalents in order for methane, nitrous oxide, and CFC-12, respectively.
To know more about Global Warming Potential, visit:
https://brainly.com/question/6595067
#SPJ1
I need help with this

Answers
Answer:
2 nitrogen molecules are present
1 oxygen molecule is present
1 n2o molecules are present
Which of the following items are made from renewable resources? Select the two correct answers. (1 point)
Responses
plastic fork
plastic fork
metal can
metal can
leather jacket
leather jacket
electronics
electronics
printer paper
Answers
A leather jacket and printer paper are examples of items that can be made from renewable resources, while plastic forks, metal cans, and electronics are not considered renewable due to their reliance on non-renewable materials and processes. Option C, E
The two correct answers that are made from renewable resources are:
C) Leather jacket: Leather is derived from animal hides, which are a byproduct of the meat industry. As long as there is a sustainable and responsible approach to animal farming, the production of leather can be considered renewable. The hides are obtained from animals that are raised for meat consumption, and their use in leather production helps reduce waste.
E) Printer paper: Printer paper can be made from various sources, including trees, bamboo, and recycled paper fibers. If the paper is sourced from sustainably managed forests or from fast-growing plants like bamboo, it can be considered renewable. Additionally, the use of recycled paper fibers reduces the demand for materials and promotes a more circular economy.
The other options, A) plastic fork, B) metal can, and D) electronics, are not made from renewable resources:
A) Plastic fork: Plastics are typically derived from fossil fuels, which are non-renewable resources. The production of plastic involves the extraction and processing of petroleum or natural gas, both of which are finite resources.
B) Metal can: Metal cans are predominantly made from aluminum or steel. While these metals can be recycled, their initial production requires the extraction of raw materials from the Earth, which is not a renewable process.
D) Electronics: Electronics are made from a wide range of materials, including metals, plastics, and various chemical compounds. The production of electronics involves the extraction of raw materials, many of which are non-renewable resources.
Option C and E.
For more such questions on renewable resources visit:
https://brainly.com/question/27734408
#SPJ8
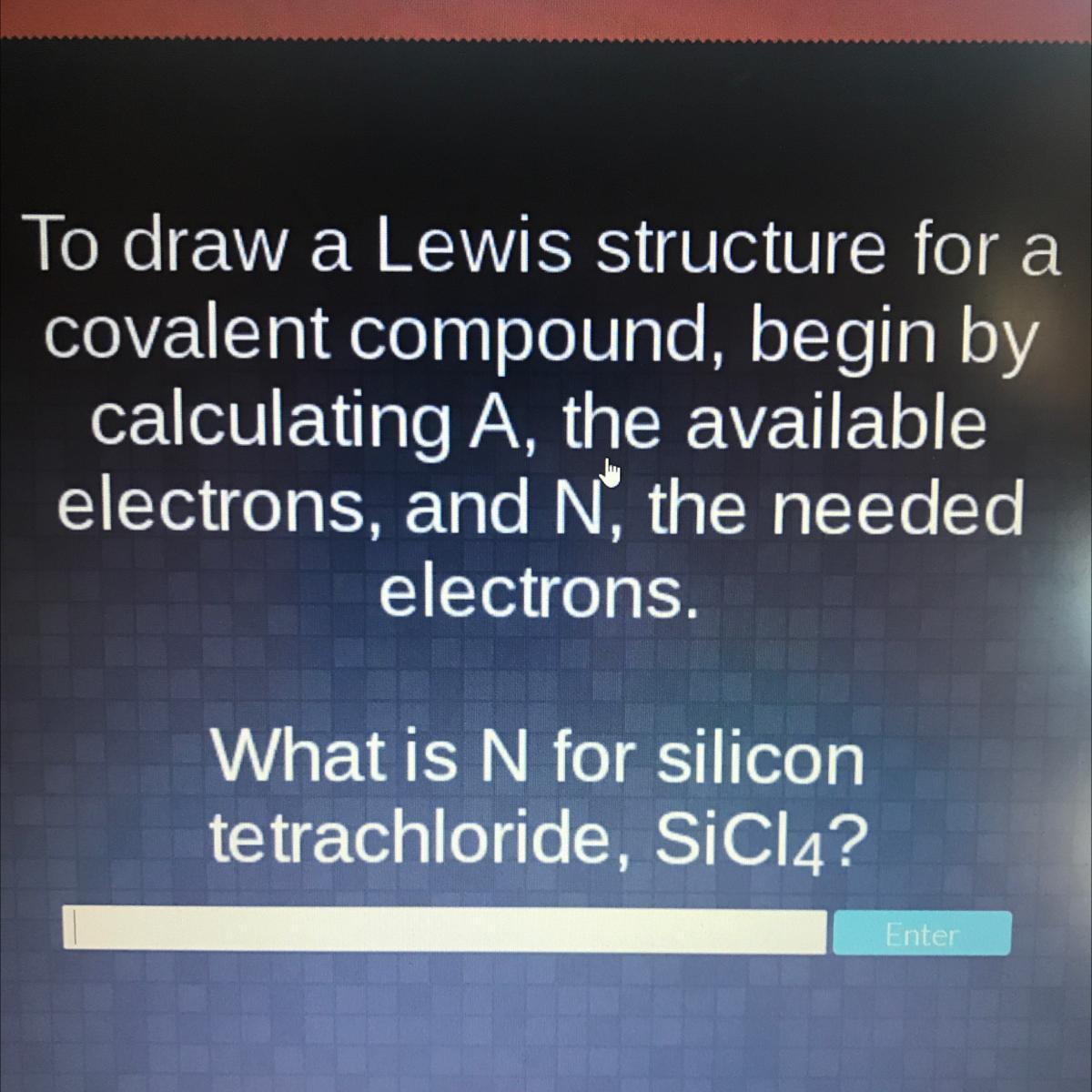
What is N for silicon tetrachloride, SiCl4?
Answers
MUST BE CORRECT AND DONE ASAP 100 POINTS
NOT AI WRITTEN

Answers
About two electrons can have that quantum number.
Mg has the highest IE2 while Al has the highest IE3
The order of increasing atomic radius is; Cl> Te^2- >Te > S
What are the quantum numbers?Quantum numbers are a set of four parameters that are used to describe the state of an electron in an atom. They describe the energy, orbital shape, orientation, and spin of an electron in an atom. The four quantum numbers are:
Principal quantum number (n): This quantum number describes the energy level of an electron in an atom. It can take on any positive integer value, with higher values indicating higher energy levels. The principal quantum number determines the size of the electron's orbital.
Azimuthal quantum number (l): This quantum number describes the shape of the electron's orbital. It can take on values from 0 to (n-1).
Magnetic quantum number (m): This quantum number describes the orientation of the electron's orbital in space. It can take on values from -l to +l.
Spin quantum number (s): This quantum number describes the intrinsic angular momentum, or "spin," of the electron. It can have a value of +1/2 or -1/2, indicating the direction of the electron's spin.
Learn more about quantum number:https://brainly.com/question/16977590
#SPJ1
How many grams of carbon dioxide are produced by complete combustion of 5 grams of c5h10
Answers
Answer:
15.4 g
Explanation:
The reaction that takes place is:
2C₅H₁₀ + 15O₂ → 10CO₂ + 10H₂OFirst we convert 5 grams of C₅H₁₀ into moles, using its molar mass:
5 g ÷ 70 g/mol = 0.07 mol C₅H₁₀Then we convert C₅H₁₀ moles into CO₂ moles, using the stoichiometric coefficients of the reaction:
0.07 mol C₅H₁₀ * \(\frac{10molCO_2}{2molC_5H_{10}}\) = 0.35 mol CO₂Finally we convert 0.35 moles of CO₂ into grams:
0.35 mol CO₂ * 44 g/mol = 15.4 gWith 21 g of Zinc, and 7 g of CuCl2, how much ZnCl2 is made in grams?
Answers
Answer: 7.07 grams
Explanation:
To calculate the moles :
\(\text{Moles of solute}=\frac{\text{given mass}}\times{\text{Molar Mass}}\)
\(\text{Moles of} zinc=\frac{21g}{65g/mol}=0.32moles\)
\(\text{Moles of} CuCl_2=\frac{7g}{134g/mol}=0.052moles\)
\(Zn+CuCl_2\rightarrow Cu+ZnCl_2\)
According to stoichiometry :
1 mole of \(CuCl_2\) require 1 mole of \(Zn\)
Thus 0.052 moles of \(CuCl_2\) will require=\(\frac{1}{1}\times 0.052=0.052moles\) of \(Zn\)
Thus \(CuCl_2\) is the limiting reagent as it limits the formation of product and \(Zn\) is the excess reagent.
As 1 mole of \(CuCl_2\) give = 1 mole of \(ZnCl_2\)
Thus 0.052 moles of \(CuCl_2\) give =\(\frac{1}{1}\times 0.052=0.052moles\) of \(ZnCl_2\)
Mass of \(ZnCl_2=moles\times {\text {Molar mass}}=0.052moles\times 136g/mol=7.07g\)
Thus 7.07 g of \(ZnCl_2\) will be produced from the given masses of both reactants.
An energy of 4.50x10^-19 J/photon was released when an electron drops to a lower energy state, what is the wavelength of the photon? What color does this energy correspond to?
Answers
4.41 × 10⁻⁴⁵m is the wavelength of the photon and the energy correspond to red color.
What do you mean by the wavelength ?The term wavelength is defined as the distance between two identical points that are adjacent crests and troughs.
The SI unit of wavelength is metre mostly represented as m.
The wavelength is mostly represented by λ is the Greek letter lambda.
Given:
E = 4.50x10⁻¹⁹ J/photon
h = Planck constant = 6.626 × 10⁻³⁴
ν = ?
E = hν
ν = E/h
By substituting the values in above question and we get,
= 4.50x10^-19 / 6.626 × 10⁻³⁴
= 0.679 × 10⁻¹⁵
c = 3 × 10⁸
E = hc/λ
λ = hc/E
By substituting the values in above question and we get,
λ = 6.626 × 10⁻³⁴ × 3 × 10⁸ / 4.50x10⁻¹⁹
λ = 4.41 × 10⁻⁴⁵m
Thus, the wavelength of the photon is 4.41 × 10⁻⁴⁵m and color does this energy correspond to red.
To learn more about the wavelength, follow the link;
https://brainly.com/question/13533093
#SPJ9
why is the sun earth and moon system important
Answers
The Sun-Earth-Moon system is important because it sustains life on Earth, regulates Earth's climate, and influences natural phenomena like tides.
The Sun-Earth-Moon system plays a vital role in supporting and sustaining life on Earth. The Sun is the primary source of energy for our planet, providing heat and light necessary for photosynthesis, the process by which plants convert sunlight into food and oxygen. Sunlight is also crucial for maintaining Earth's temperature and driving weather patterns.
The Moon, as Earth's only natural satellite, contributes to several essential functions. Its gravitational pull creates the tides, which influence coastal ecosystems and shape coastal landscapes.
The Moon's orbit also stabilizes Earth's axial tilt, providing a stable climate for life to thrive. Additionally, the Moon's phases have cultural and historical significance, influencing human activities such as agriculture, navigation, and calendar systems.
The Sun-Earth-Moon system's interactions are responsible for natural phenomena like eclipses, both solar and lunar, which have fascinated humans throughout history and continue to be important for scientific study and exploration.
Understanding these celestial events enhances our knowledge of astrophysics and helps us comprehend the vastness and complexity of the universe.
Furthermore, the study of the Sun-Earth-Moon system provides insights into celestial mechanics, orbital dynamics, and the broader field of planetary science. By examining the interplay between these celestial bodies, scientists can gain a deeper understanding of Earth's place in the universe and explore potential habitable conditions on other celestial bodies.
Overall, the Sun-Earth-Moon system is of immense importance as it sustains life, regulates climate, influences natural phenomena, and provides a platform for scientific exploration and discovery.
For more question on climate visit:
https://brainly.com/question/12801279
#SPJ8
How many moles of N are in 0.217 g of N2O ?
Answers
1 x 10^-2.
There are approximately 0.00493 moles of N in 0.217 g of N2O.
Explanation:To determine the number of moles of N in 0.217 g of N2O, we need to convert the mass of N2O to moles using the molar mass of N2O, which is 44.0128 g/mol. We can use the formula:
moles = mass / molar mass
So, moles of N = 0.217 g / 44.0128 g/mol = 0.00493 mol. Therefore, there are approximately 0.00493 moles of N in 0.217 g of N2O.
Learn more about moles of N in N2O here:https://brainly.com/question/4104576
#SPJ2
Determine the limiting reactant (LR) and the mass (in g) of nitrogen that can be formed from 50.0 g N 2O 4 and 45.0 g N 2H 4. Some possibly useful molar masses are as follows: N 2O 4 = 92.02 g/mol, N 2H 4 = 32.05 g/mol.
N 2O 4( l) + 2 N 2H 4( l) → 3 N 2( g) + 4 H 2O( g)
a) LR = N2O4, 45.7 g N2 formed
b) LR = N2O4, 105 g N2 formed
c) LR = N2H4, 13.3 g N2 formed
d) LR = N2H4, 59.0 g N2 formed
e) No LR, 45.0 g N2 formed
Answers
Answer:
Option A. LR = N2O4, 45.7g N2 formed
Explanation:
The balanced equation for the reaction is given below:
N2O4(l) + 2N2H4(l) → 3N2(g) + 4H2O(g)
Next, we shall determine the masses of N2O4 and N2H4 that reacted and mass of N2 produced from the balanced equation. This is illustrated below:
Molar mass of N2O4 = 92.02 g/mol
Mass of N2O4 from the balanced equation = 1 x 92.02 = 92.02 g
Molar mass of N2H4 = 32.05 g/mol
Mass of N2H4 from the balanced equation = 2 x 32.05 = 64.1g
Molar mass of N2 = 2x14.01 = 28.02g/mol
Mass of N2 from the balanced equation = 3 x 28.02 = 84.06g
Summary:
From the balanced equation above,
92.02g of N2O4 reacted with 64.1g of N2H4 to produce 84.06g of N2.
Next, we shall determine the limiting reactant. This can be obtained as follow:
From the balanced equation above,
92.02g of N2O4 reacted with 64.1g of N2H4.
Therefore, 50g of N2O4 will react with = (50 x 64.1)/92.02 = 34.83g of N2H4.
From the calculations made above, we can see that only 34.83g out 45g of N2H4 is required to react completely with 50g of N2O4.
Therefore, N2O4 is the limiting reactant and N2H4 is the excess reactant.
Finally, we shall determine the mass of N2 produced from the reaction.
In this case the limiting reactant will be used as it will produce the maximum yield of N2 since all of it is used up in the reaction.
The limiting reactant is N2O4 and the mass N2 produced can be obtained as illustrated below:
From the balanced equation above,
92.02g of N2O4 reacted to produce 84.06g of N2.
Therefore 50g of N2O4 will react to produce = (50 x 84.06)/92.02 = 45.7g of N2.
Therefore, 45.7g of N2 were produced from the reaction.
At the end of the day,
The limiting reactant is N2O4 and 45.7g of N2 were produced from the reaction.
Please show all of your work, I need to understand all steps:
A student collected time (t) and concentration ([A]) data at
295 K for the reaction 2A→B. These time and concentration data are shown in the table to the right. The student then plotted graphs of [A] versus t (Figure 1) , ln[A] versus t (Figure 2) , and 1/[A] versus t (Figure 3) .
Figure 1:
t (s) (A)(M) ln(A) 1/ (A)
0.00 0.500 −0.693 2.00
20.0 0.389 −0.944 2.57
40.0 0.303 −1.19 3.30
60.0 0.236 −1.44 4.24
80.0 0.184 −1.69 5.43
i.) What is the rate of the reaction?
ii.) What is the value of the rate constant for this reaction?
iii.)
Next, the student ran the same reaction at a different temperature and measured a different reaction rate, with the following results:
T(K) k(s-1)
295 0.0125
325 0.0357
What is the activation energy of this reaction?
Express your answer to three significant figures and include the appropriate units.



Answers
Answer:
a because the x and y value is the same
Does this sound catchy or invite you to read more? "GMOs and pesticides are general amongst farmers for generations and should not be that common." If not please help me.
Answers
Answer:
It sounds fine, but it may be a bit too long. It's difficult to shorten things like this, but getting more straight to the point would give it that "catchy" feel.
Explanation: