Consider the reaction: 2 H Br(g) + H2(g) + Br2(g) a. In the first 24 s of this reaction, the concentration of HBr dropped from 0.792 M to 0.455 M. What is the average rate of the reaction during this time interval? b. If the volume of the reaction vessel was 1.69 L, what amount of Br2 (in moles) was formed during the first 11 s of the reaction?
Answers
a. the average rate of the reaction is (0.337 M) / (24 s) = 0.01404 M/s.
b. amount of Br2 (in moles) was formed during the first 11 s of the reaction 0.13040 moles of Br2.
a. To calculate the average rate of the reaction during the first 24 seconds, we can use the formula: average rate = (change in concentration) / (change in time).
The concentration of HBr dropped from 0.792 M to 0.455 M, so the change in concentration is 0.792 - 0.455 = 0.337 M. The change in time is 24 seconds. Therefore, the average rate of the reaction is (0.337 M) / (24 s) = 0.01404 M/s.
b. To determine the amount of Br2 formed during the first 11 seconds of the reaction, we first need to find the change in HBr concentration during this time.
Using the average rate we calculated, we can find the change in HBr concentration during 11 seconds: (0.01404 M/s) * (11 s) = 0.15444 M.
Since the reaction ratio is 2 HBr to 1 Br2, the change in Br2 concentration is half of the change in HBr concentration: 0.15444 M / 2 = 0.07722 M. Now, multiply the change in Br2 concentration by the volume of the reaction vessel
(1.69 L) to find the amount of Br2 formed in moles: 0.07722 M * 1.69 L = 0.13040 moles of Br2.
To know more about reaction refer here: https://brainly.com/question/17434463#
#SPJ11
Related Questions
What would you predict, the solubility of KHT (solid) in pure water compared with the solubility of KHT (solid) in a 0.1 M KCl solution, which one will be higher? Explain your answer.
Answers
The solubility of KHT (solid) in pure water compared with the solubility of KHT (solid) in a 0.1 M KCl solution is predicted to be higher in the 0.1 M KCl solution. This is because the KCl solution has a higher ionic strength, increasing the solubility of ionic compounds like KHT.
Let's understand this in detail:
What is solubility?
Solubility is defined as the ability of a substance to dissolve in a particular solvent under certain conditions. It measures the maximum amount of solute that can be dissolved in a given amount of solvent at a particular temperature, pressure, and other conditions.
Solubility of KHT in pure water:
KHT (Potassium hydrogen tartrate) is a weak acid salt that has low solubility in pure water. The solubility of KHT in pure water is affected by various factors such as temperature, pH, and pressure. The solubility of KHT in pure water is around 4.4 g/L at room temperature.
Solubility of KHT in 0.1 M KCl solution: The solubility of KHT in a 0.1 M KCl solution is predicted to be higher than in pure water. KCl is an ionic salt dissociating in water to produce K+ and Cl- ions. The presence of KCl increases the ionic strength of the solution. This ionic strength improves the solubility of other ionic compounds, such as KHT. KHT has a higher solubility in a 0.1 M KCl solution than in pure water due to this reason.
#SPJ11
Learn more about solubility: Explain how you would find the solubility of a solute https://brainly.com/question/23946616
please help asap in 10 mins
What are the conditions necessary for electro-chemical corrosion to occur?
Answers
Answer:
Presence of an Electrolyte
Metal Surface
Oxygen or Other Oxidizing Agent
Difference in Potential
Electrochemical Pathway
Explanation:
in an adiabatic process oxygen gas in a container is compressed along a path that can be described by the following pressure p, in atm, as a function of volume v, in liters:h
Answers
In an adiabatic process, the relationship between pressure and volume is given by the equation pv^γ = constant, where γ is the heat capacity ratio. This equation describes the path along which the oxygen gas is compressed in the container.
The adiabatic process implies that no heat is exchanged with the surroundings. Therefore, the internal energy of the gas changes solely due to work done on it. As the gas is compressed, its volume decreases and the pressure increases. The specific value of γ depends on the gas being compressed.
For oxygen gas, γ is approximately 1.4. This adiabatic process can be visualized on a graph by plotting the pressure on the y-axis and the volume on the x-axis. The path of the compression will be a curve that decreases as the volume decreases.
To know more about adiabatic visit:-
https://brainly.com/question/33498093
#SPJ11
a certain weak base has a Kb of 7.10×10−7. what concentration of this base will produce a ph of 10.04? concentration:
Answers
The Concentration is 0.055 M
Solution:
pOH = 1/2(pKb - logC)
pOH = 14-pH = 14-10.31 = 3.69
pKb = -log kb = -log 7.100 x 10-7 = 64
3.69 = 1/2(6.12-log C)
Concentration = 0.055 M
Solution concentration is defined as the amount of solute present in a given volume of solution. It can be expressed as mass x mass percent of solution = mass of solute mass in solution × 100. mass x volume percent of solution = mass of solute mass in solution × 100.
By focusing, you can make better use of your resources and tackle problems more efficiently. When you concentrate, you are less likely to miss important information. Staying focused makes it easier to remember things. Focus means control of attention. It is the ability to focus the mind on a subject or an object.
Learn more about The Concentration here:- https://brainly.com/question/17206790
#SPJ4
8. If a neutral atom contains 18 protons, how many electrons does it have?A. 9B. 36C. 18
Answers
Answer:
C. 18
Explanation:
The number of protons of an element, is represented by the Atomic number. It also represents the number of electrons in a neutral atom.
So, in this case, if a neutral atom contains 18 protons, it will have 18 electrons.
T/F. Based on the irradiation handout, you interact with irradiated plastic ware when you donate blood
Answers
The given statement "Based on the irradiation handout, you interact with irradiated plastic ware when you donate blood" is TRUE because when you donate blood, you do interact with irradiated plastic ware.
What's irradiation?Irradiation is a process used to sterilize medical equipment, including plastic blood collection bags and tubing, to ensure the safety of blood transfusions.
This process involves exposing the plastic ware to ionizing radiation, which eliminates any potential contaminants, such as bacteria or viruses, without affecting the blood itself.
By using irradiated plastic ware during blood donation, healthcare professionals minimize the risk of infections and ensure that the collected blood remains safe and suitable for transfusion to patients in need.
Learn more about irradiation at
https://brainly.com/question/28199793
#SPJ11
Which of the following molecules has a trigonal planar shape? A P E X
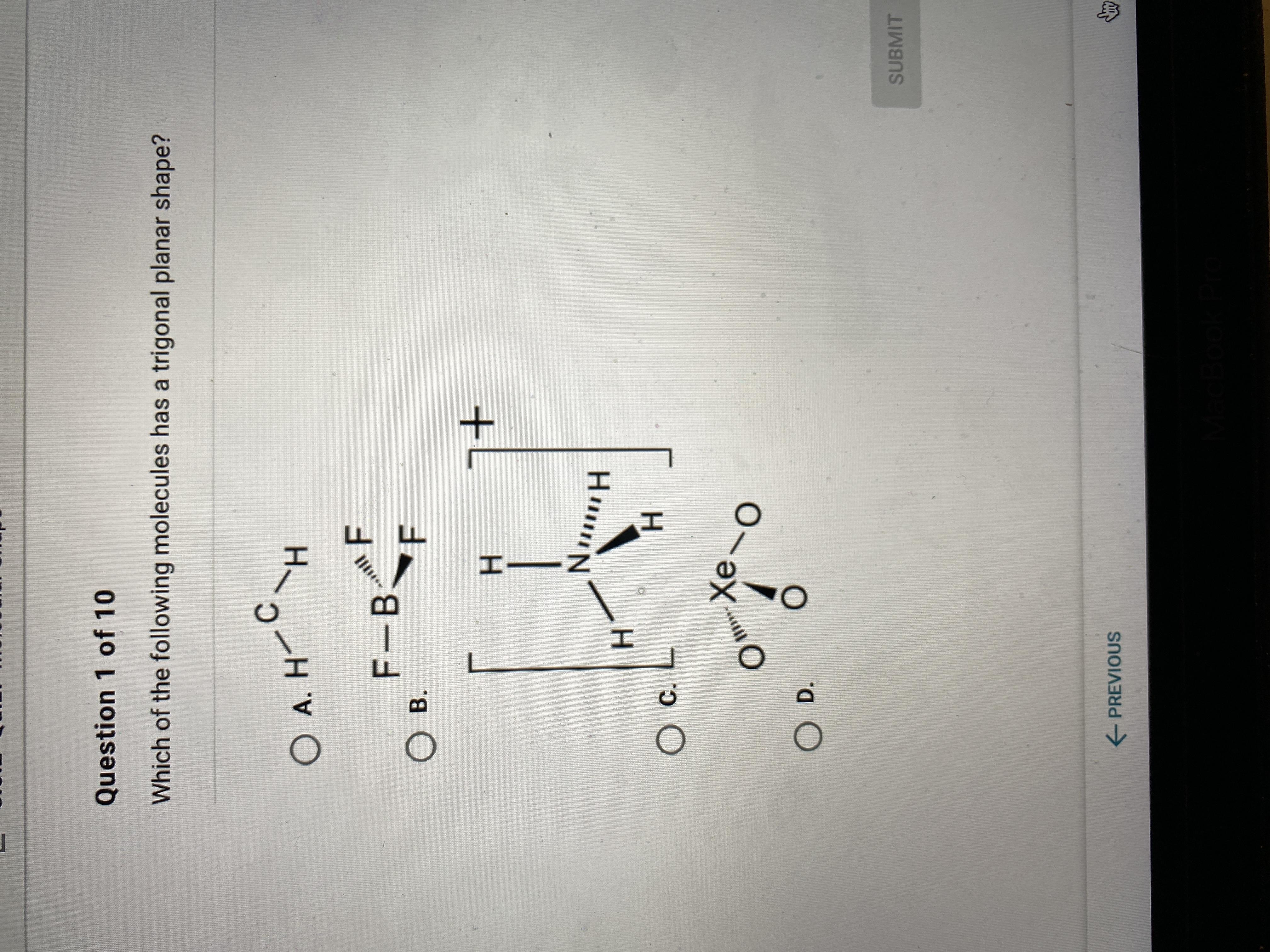
Answers
Answer:
B. BF3
Explanation:
I did summoning jutsu on it and got it correct
How do you find the unknown compound in organic chemistry?
Answers
The unknown compounds are among a restricted set of substances that are provided for you in ascending mp and bp order. Applying your experimental results to these lists will help you narrow down the list of potential chemicals.
Compounds can be recognized by two tests, including
1. Physical assessment
2. Chemical assessment
Physical assessment
This is dependent on outward manifestations and qualities including State, Color, Texture, Smell (odor), Taste, and Feel.
Physical characteristics such as solubility, crystalline or amorphous nature Refractive index, melting point, and boiling point
pH, conductivity, functional groups, and other chemical assessment criteria. utilizing analytical approaches such as spectroscopy analysis and chromatography analysis.
To know more about unknown compound visit
https://brainly.com/question/28456436
#SPJ4
If water’s density is 1.0 g/mL, then would the perfume be more or less dense than water? Would the perfume float on top or sink to the bottom of the water?
Help me out rq!!!!!
Answers
Answer:
usually the perfumes are made of aromatic hydrocarbons invloving
cetone, ethanol, benzaldehyde, formaldehyde, limonene, methylene chloride, camphor, ethyl acetate, linalool and benzyl alcohol. which have density lower than the water hence they will float on the top of the water.
Hope this helps you
Explanation:
which one is not one of the four most abundant elements in life?
Answers
TRUE / FALSE. cesium hydroxide, csoh, is dissolved in water to make up a solution that is 0.0325m in csoh. what is the ph of the solution at 25.0∘c? round the answer to three significant figures.
Answers
The p h of the solution at 25.0∘c.
To determine the pH of the solution, we need to first calculate the concentration of hydroxide ions (OH-) in the solution, and then use that value to find the pOH. Finally, we can calculate the pH using the equation pH + pOH = 14.
Cesium hydroxide (C s OH) is a strong base that dissociates completely in water. It releases one hydroxide ion (OH-) for each Cs OH molecule.
Given that the solution is 0.0325 M in Cs OH, the concentration of OH- ions is also 0.0325 M.
Next, we can calculate the pOH:
pOH = -log[OH-] = -log(0.0325) ≈ 1.49
Finally, we can calculate the pH:
pH = 14 - pOH ≈ 14 - 1.49 ≈ 12.51
Therefore, the pH of the solution at 25.0 °C is approximately 12.51 (rounded to three significant figures).
To know more about ions:
https://brainly.com/question/32229428
#SPJ4
How many grams of water are produced when 35.8 grams of Calcium hydroxide reacts with lots of Hydrochloric acid?
Answers
Are produced 72 grams of water in this reaction.
Mole calculationTo find the value of moles of a product from the number of moles of a reactant, it is necessary to observe the stoichiometric ratio between them:
\(Ca(OH)_2 + 2HCl = > 2H_2O + CaCl_2\)
Analyzing the reaction, it is possible to see that the stoichiometric ratio is 1:2, so we can perform the following expression:
\(MM_{Ca(OH)_2} = 74.1g/mol\)
\(MM = \frac{g}{mol}\)
\(74.1 = \frac{35.8}{mol}\\mol = 2\)
So, if there are 2 mols of Ca(OH)2:
Ca(OH)2 | H2O
\(\frac{1mol}{2mol} =\frac{2mol}{xmol}\)
\(x = 4mol\)
Finally, just find the number of grams of water using your molar mass:
\(MM_{H_2O} = 18g/mol\)
\(18= \frac{m}{4}\\m = 72g\)
So, 72 grams are produced of water in this reaction.
Learn more about mole calculation in: brainly.com/question/2845237
1. What is the major determining factor in soil formation? Define this factor and explain how it influences soil formation.
PLEASE HELP ME PLEASE
Answers
Answer:
Temperature and precipitation
Explanation:
They determine how quickly weathering will be, and what kind of organic material may be available on the inside of the soils.
Temperature and precipitation is the major determining factor in soil formation. They define the type of organic material that can be present inside the soils as well as how rapidly weathering will occur.
What is soil?The bioactive, porous media that has grown in the top layer of the Earth's crust is known as soil. Being a source of water as well as nutrients, a filter for harmful wastes, a site for their breakdown, and a participant inside the cycle of elements such as carbon through the planet's ecosystem, soil constitutes one of the main substrates of life on Earth.
It has changed as a result of weathering processes influenced by geographical, geologic, biological, and climatic factors. A practical understanding of soils including their management has also developed out of need since the development of agriculture with forestry in the eighth millennium BCE. Temperature and precipitation is the major determining factor in soil formation. They define the type of organic material that can be present inside the soils as well as how rapidly weathering will occur.
Therefore, temperature and precipitation is the major determining factor in soil formation.
To know more about soil, here:
https://brainly.com/question/27588666
#SPJ3
There are three stable isotopes of magnesium. Their masses are 23.9850, 24.9858, and 25.9826 u. If the average atomic mass of magnesium is 24.3050 u and the natural abundance of the lightest isotope is 78.99%, what are the natural abundances of the other two isotopes?
Answers
Answer:
²⁵ Mg = 10.00%
²⁶ Mg = 11.0%
Explanation:
Given that:
Magnesium ²⁴Mg has an abundance of 78.99%
Hence, (100 - 78.99)% = 21.01%
21.01% is the abundance of ²⁵ Mg and ²⁶ Mg
Suppose,
²⁵ Mg = x
²⁶ Mg = (21.01 - x)%
Then;
avg. atomic mass = \(\dfrac{78.99}{100 }(23.9850)+\dfrac{x}{100}(24.9858) +\dfrac{21.01-x}{100}(25.9826)\)
where the avg. atomic mass = 24.3050
∴
\(24.3050 \times 100 = {78.99}(23.9850)+\dfrac{x}{100}(24.9858) +\dfrac{21.01-x}{100}(25.9826)\)
2430.5 = 2440.46915 -0.9968 x
0.9968x = 2440.46915 - 2430.5
0.9968x = 9.96915
x = 9.96915/0.9968
x = 10.00%
∴ Recall that:
²⁵ Mg = x
²⁶ Mg = (21.01 - x)%
²⁵ Mg = 10.00%
²⁶ Mg = (21.01 - 10.00)%
²⁶ Mg = 11.0%
The natural abundances of the other two isotopes, that is, Mg-25 and Mg-26 are 10% and 11.01%.
What is Natural abundance?It is the abundance of isotopes of a chemical element as naturally found on a planet.
Based on the given information:
The three stable isotopes of Mg are Mg-24, Mg-25 and Mg-26. The natural abundance of the lightest isotope, that is, Mg-24 is 78.99%. The masses of the isotopes are 23.9850 u, 24.9858 u and 25.9826 u. The average atomic mass of Mg is 24.3050 u.Now the natural abundance of all the isotopes of Mg is 100%.
Mg-24 + Mg-25 + Mg-26 = 100
Mg-25 + Mg-26 = 100-78.99
Mg-25 + Mg-26 = 21.01
Now let us assume that Mg-25 is x% then Mg-26 will be (21.01% -x).
Now,
Average atomic mass of Mg = [(Natural abundance of Mg-24) × (isotopic mass of Mg-24) + (Natural abundance of Mg-25) × (Isotopic mass of Mg-25) + (Natural abundance of Mg-26) × (Isotopic mass of Mg-26)]
Putting the values we get,
24.3050 amu = (78.99% × 23.9580 amu) + (x% × 24.9858 amu) + (21.01% - x%) × 25.9826 amu
24.3050 amu = 18.9458 + 24.9858 × x + 5.4589 - (25.9826x)
24.3050 = 24.4047 - (0.9968x)
0.9968x = 24.4047 - 24.3050
x = 0.1000
x = 10%
The natural abundance of Mg-25 is 10%.
The natural abundance of Mg-26 = 21.01% - x% = 21.01% - 10% = 11.01%.
Thus, the natural abundances of the other two isotopes are 10% and 11.01%.
Find out more information about natural abundances here:
https://brainly.com/question/4880347
(b) Liquids and gases flow because…?
Answers
The molecules of gases and liquids are present far apart from each other. In other words, they have more gaps or intermolecular spaces. Due to the large intermolecular forces, the intermolecular attractions are very less and thus liquids and gases can flow.
What is an expression of Dalton's law (k = constant)?

Answers
Answer:
I believe it's B: Ptotal=P1+P2+P3+...
Explanation:
4. Which part of the eye can change shape and
help form an image?
A 1
B 2
C 3
D 4

Answers
i believe the answer is 2, good luck
define a primary source and secondary source
Answers
Answer:
primary source: documents the result of original researche.
: is written by those who have conducted researche.
: includes firsthand information about their methodologies, data, results or conclusions.
Secondary source: summarizes, compares, critiques or interprets the primary source.
Explanation:
Primary source is the research articles published in scholarly, peer reviewed journal.
Secondary source is the summarises, critiques or interpretations of primary source.
he long run equilibrium condition for perfect competition is:
a. P=AVC=MR=MC.
b. Q=AVC=MR=MC.
c. Q=ATC=MR=MC.
d. P=ATC=MR=MC.
Answers
Option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
The long-run equilibrium condition for perfect competition is that price (P) is equal to average total cost (ATC), which is also equal to marginal cost (MC), and marginal revenue (MR).
Option (d), P=ATC=MR=MC, best represents the long-run equilibrium condition for perfect competition. In perfect competition, firms operate at the minimum point of their average total cost curve, where price equals both average total cost and marginal cost. This condition ensures that firms are earning zero economic profit and are producing at an efficient level.
In the long run, if firms are earning economic profit, new firms will enter the market, increasing competition and driving prices down. Conversely, if firms are experiencing losses, some firms may exit the market, reducing competition and causing prices to rise. This process continues until firms reach a state where price equals average total cost, marginal cost, and marginal revenue, ensuring a long-run equilibrium.
Therefore, option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
Know more about Equilibrium here:
https://brainly.com/question/30694482
#SPJ11
Use Table B in your Student Guide to answer the questions about ion concentrations.
A solution with a pH = 13 has approximately how many moles of OH– ions per liter?
0.1
How many moles of H+ would this same solution have per liter?
0.000000000000.1
⇒ 0.0000000000001
(Use the decimal form of your answer.)
A different solution with an H+ concentration of 1.0 × 10–4 would have a pH =
4
.
Answers
1. The amount of OH¯ present in the solution is 0.1 mol/L
2. The amount of H⁺ present in the solution is 0.0000000000001 mol/L
3. The pH of the solution with H⁺ concentration of 1×10⁻⁴ mol/L is 4
1. How to determine the amount of OH¯ in the solutionWe'll begin by obtaining the pOH of the solution
pH = 13pOH =?pH + pOH = 14
13 + pOH = 14
Collect like terms
pOH = 14 - 13
pOH = 1
Finally, we can determine the OH¯
pOH = 1OH¯ =?pOH = –Log [OH¯]
1 = –Log [OH¯]
Multiply through by –1
–1 = Log [OH¯]
Take the anti-log of –1
[OH¯] = anti-log of (–1)
[OH¯] = 0.1 mol/L
2. How to determine the amount of H⁺pH = 13H⁺ =?pH = –Log [H⁺]
13 = –Log [OH¯]
Multiply through by –1
–13 = Log [H⁺]
Take the anti-log of –13
[H⁺] = anti-log of (–13)
[H⁺] = 0.0000000000001 mol/L
3. How to determine the pHH⁺ = 1×10⁻⁴ mol/LpH =?pH = –Log [H⁺]
pH = –Log 1×10⁻⁴
pH = 4
Learn more about pH:
https://brainly.com/question/3709867
#SPJ1
Which of these is a measure of the average kinetic energy of the molecules of a material? A.heat B.temperature C.thermal energy D.bonding energy
Answers
Answer:
b temperature
Explanation:
Temperature is a measure of the average kinetic energy of the particles in a substance
How many moles are in 6.3x1054 molecules of Ca(C2H202)2?
Answers
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance in that sample, measured in moles. It is the mass of 1 mole of the substance or 6.022×10²³ particles, expressed in grams.
Can an asteroid be pure metal? (a) No; all asteroids contain rock. (b) Yes; it must have formed where only metal could condense in the solar nebula. (c) Yes; it must have been the core of a shattered asteroid.
Answers
When an asteroid enters the surface of the earth, it gets shattered. The core of a shattered asteroid contains pure metals such as iron and nickel.
Asteroids are present in space in the form of large rocks. The rocks are made up of minerals and metals. Asteroids revolve around the sun in an orbit. Asteroids are also known as small planets. Therefore, these asteroids are made up of materials similar to that of a planet.
Asteroids can be divided into 3 categories:
1) C- Type:- These asteroids contain clays and stony particles.
2) S- Type:- The asteroid will contain silicates and a composition of nickel-iron metal.
3) M-Type:- These have nickel and iron metals.
Along with these materials, dirt and dust are also found inside these small planets.
The correct answer is (C).
To know more about asteroids, visit: https://brainly.com/question/7250354
#SPJ1
what happpens when nitrogen fills its valence shell?
A. Three electrons are lost, creating N+3
B. Three electrons are gained, creating N-3
C. Three electrons are gained creating N+3
D. Three electrons are lost, creating N-3
Answers
Nitrogen needs to gain 3 electrons to have a full valence shell so whenever its given those three it becomes N-3
Polyelectrolytes are typically used to separate oil and water in industrial applications. The separation process is dependent on controlling the pH. Fifteen (15) pH readings of wastewater following these processes were recorded. Is it reasonable to model these data using a normal distribution? 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 10.0 10.5 7.6 11.4 11.4 10.0 Yes, it passes the "fat pencil" test. Therefore, a normal distribution is a reasonable model. No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O Yes, it passes the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O No, it does not pass the "fat pencil" test. Therefore, a normal distribution is a reasonable model.
Answers
No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. Option B is the correct answer.
The "fat pencil" test is a quick visual check to determine if a dataset can be reasonably approximated by a normal distribution. In this case, the pH readings of wastewater show a significant deviation from a normal distribution. The presence of several low pH values (1.0) and a few high pH values (10.0, 10.5, 11.4) indicate a non-normal distribution with skewness and potential outliers. Therefore, it is not reasonable to model these data using a normal distribution.
Option B is the correct answer.
You can learn more about normal distribution at
https://brainly.com/question/4079902
#SPJ11
10. There are two bears that both have the same proteins for ear shape in their cells. The bears have
different parents. What can you say about the bears' ear shape?
Answers
Answer:
They will have the same ear shape since their proteins connect in the same ways to determine ear shape.
Explanation:
I majored in Chemistry
Answer:
They will have the same ear shape since their proteins connect in the same ways to determine ear shape.
Explanation:
what is the unique characterization of a ph buffer?
Answers
electron dot structure of sulphur ??
hand drawn plz
Answers
The dot electron structure of sulfur shows six electrons contains in its outermost shell.
The dot electron structure is a representation that shows the number of electrons that are found on the valence shell of an atom. The valence shell is the outermost shell of the atom that contains the valence electrons present in the atom.
A good electron dot structure contains;
The symbol of the element The number of valence electron shown as dots.Hence, the dot electron structure of sulfur shows six electrons contains in its outermost shell.
Learn more about electron dot structure: https://brainly.com/question/5414444

27.5 cm³ of a solution of NaOH neutralizes 25.0cm³ of 0.5 MHCL solution. Calculate the
concentration of NaOH in
b. gdm
a. Moldm-3
Answers
a)The concentration of NaOH in g/dm³ is approximately 18.18 g/dm³, and b)The concentration in mol/dm³ is approximately 0.4545 mol/dm³.
a)To calculate the concentration of NaOH in g/dm³ (grams per cubic decimeter) and mol/dm³ (moles per cubic decimeter), we need to know the amount of NaOH used in the reaction and the volume of the NaOH solution.
From the given information, we have:
Volume of NaOH solution = 27.5 cm³
Volume of HCl solution = 25.0 cm³
Molarity of HCl solution = 0.5 M
Since the reaction between NaOH and HCl is a 1:1 stoichiometric ratio, the moles of NaOH used can be determined from the moles of HCl used:
Moles of HCl = Molarity × Volume = 0.5 M × 25.0 cm³ = 12.5 mmol (millimoles)
Since the moles of NaOH used is also equal to the moles of HCl, we have:
Moles of NaOH = 12.5 mmol
b)To calculate the concentration of NaOH in g/dm³, we need to convert moles to grams using the molar mass of NaOH, which is approximately 40 g/mol:
Mass of NaOH = Moles × Molar mass = 12.5 mmol × 40 g/mol = 500 g
Now, we can calculate the concentration in g/dm³:
Concentration of NaOH (g/dm³) = Mass of NaOH / Volume of NaOH solution
= 500 g / 27.5 cm³
≈ 18.18 g/dm³
To calculate the concentration of NaOH in mol/dm³, we can use the same approach:
Concentration of NaOH (mol/dm³) = Moles of NaOH / Volume of NaOH solution
= 12.5 mmol / 27.5 cm³
≈ 0.4545 mol/dm³
Therefore, the concentration of NaOH in g/dm³ is approximately 18.18 g/dm³, and the concentration in mol/dm³ is approximately 0.4545 mol/dm³.
Know more about concentration here:
https://brainly.com/question/28464162
#SPJ8
I need help with this

Answers
2. 115
THIS IS TO REACH 20 CHARACTERS
2. 115
I hope this helps:)