Answers
How many grams of zinc (Zn) are in 32.7 g of Zn?
Answer:
32.7g
Explanation:
The mass of Zinc in the given substance is 32.7g.
Mass is the quantity of matter contained in a substance. The sum total of the amount of matter is the mass.
Since this problem gives us 32.7g of Zn then the substance will also contain 32.7g of Zinc.
Even when the Zinc is used in chemical reactions, we are expected to produce an equal amount of zinc as the product.
This way, mass is always conserved.
Related Questions
The kinetic energy of a 23.2-g object moving at a speed of 98.7 m/s is ________ J. The kinetic energy of a 23.2-g object moving at a speed of 98.7 m/s is ________ J. 0.950 145 113 1450 113000
Answers
The kinetic energy of a 23.2-g object moving at a speed of 98.7 m/s is 113.30 J. Option D
The kinetic energy of an object can be calculated using the formula: KE = (1/2)mv^2, where KE is the kinetic energy, m is the mass of the object, and v is the velocity or speed of the object.
Given:
Mass (m) = 23.2 g = 0.0232 kg
Speed (v) = 98.7 m/s
Substituting these values into the formula, we can calculate the kinetic energy:
KE = (1/2)(0.0232 kg)(98.7 m/s)^2
KE = (1/2)(0.0232 kg)(9756.09 m^2/s^2)
KE ≈ 113.30 J
Therefore, the kinetic energy of a 23.2-g object moving at a speed of 98.7 m/s is approximately 113.30 J.
It's worth noting that the question is repeated twice, but the answer remains the same. The kinetic energy of the object is determined by its mass and speed, and both calculations yield the same result. Option D
For more sucu questions on kinetic energy visit:
https://brainly.com/question/25959744
#SPJ8
Why are acids called proton donors?
Answers
The relationship between acids and bases is more aggressive than the donor/acceptor terminology implies. Bases don't passively "accept" protons; they rip hydrogen ions from acids. Acids don't "donate" hydrogen ions; they surrender them.
item 6 excessive intake of some vitamins can cause toxicity-related side effects. match the vitamin with the potential side effect when that vitamin is taken in excessive quantities.
Answers
Vomiting, headaches, wooziness, blurred vision, and even liver injury are side effects of vitamin A. Vitamin D - Tiredness, headache, sickness, and vomiting. Vitamin C: Heartburn, diarrhea, vomiting, and nausea.
What negative consequences can too much vitamin A cause?Yes, excessive amounts of some types of vitamin A can be dangerous. A strong headache, blurred vision, nausea, dizziness, muscular aches, and issues with coordination can result from taking too much preformed vitamin A, which is typically found in supplements or some medications.
Which vitamins can be harmful if consumed in large quantities?Poisonous Substance. Any component in a multivitamin supplement can be toxic in large doses, but iron or calcium pose the biggest threat. Large or toxic doses of calcium, vitamin D, and vitamin A carry additional dangers.
To know more about toxicity visit:-
https://brainly.com/question/21891087
#SPJ1
What is the mass, in grams, of 1.16 mol of water, H2O?
Answers
The mass, in grams of 1.16 mole of water, H₂O is 20.88 g
Description of moleThe mole of a substance is related to it's mass and molar mass according to the following equation
Mole = mass / molar mass
With the above formula, we can obtain the mass of H₂O
Data obtained From the questionFrom the question given above, the following data were obtained:
Mole of H₂O = 1.16 mole Molar mass of H₂O = (2×1) + 16 = 18 g/mol Mass of H₂O =? How to determine the mass of H₂OThe mass of 1.16 mole of water can be obtained as illustrated below:
Mole = mass / molar mass
Cross multiply
Mass = mole × molar mass
Mass of H₂O = 1.16 × 18
Mass of H₂O = 20.88 g
Learn more about mole:
https://brainly.com/question/13314627
#SPJ1
8) What mass of chlorine reacts with 20.0 g of iron to form iron(III) chloride?
Answers
Answer:
38.3 g
Explanation:
8) What mass of chlorine reacts with 20.0 g of iron to form iron(III) chloride?
first write, then balance the equation
Fe + Cl2-------------> FeCl3
2Fe + 3Cl2----------> 2FeCl3
2 moles of Fe require 3 moles of Cl2
the atomic mass of Fe is 56
20/56= 0.36 moles of Fe
this requires o.36 X 3/2 = 0.54 moles of Cl2
Cl2 has a molar mass of 35.5 X 2=71
1 mole of Cl2 is 71g
0.54 moles is 0.54 X 71 g= 38.3 g
The mass of chlorine reacts with 20.0 g of iron to form iron(III) chloride is 38.3 g.
What is mass?Mass is defined as a numerical representation of the fundamental characteristic of all matter, inertia.
It can also be defined as a dimensionless number used to describe the mass of a particle or item.
\(\rm 2Fe + 3Cl_2\rightarrow2FeCl_3\)
As per the reaction 3 mole of chlorine is required for 2 mole of fluorine.
Atomic mass of fluorine = 56
Moles of Fe = 20 / 56
= 0.36 moles
This require 0.36 x 3/2 = 0.54 moles of Cl₂
Molar mass of Cl = 35.5
So for Cl₂ = 2 x 35.5 = 71 moles
For 1 mole of Cl₂ = 71 moles is needed
So, for 0.54 moles = 71 x 0.54
= 38.3 g
Thus, the mass of chlorine reacts with 20.0 g of iron to form iron(III) chloride is 38.3 g.
To learn more about mass, refer to the link below:
https://brainly.com/question/19694949
#SPJ2
How does the sun cause wind?
Answers
the sun cooks the clouds the clouds pee on the earth and all the water falls to earth, pushing all the wind down that's why we have earthquakes
Locating the epicenter of an earthquake lab
Answers
Eruption triangulation is a method for locating an earthquake's epicenter.
What is epicenter?The area right above the spot in the Earth's crust where an earthquake originates or begins is known as the epicenter. It marks the spot on the Earth's surface where the earthquake's seismic waves first touchdown.
The distance between the earthquake's epicenter and a number of seismograph stations is calculated using seismograms, which are records of the ground motion brought on by an earthquake.
The following are the steps to find an earthquake's epicenter:
A minimum of three separate seismograph stations should have data collected. The epicenter's distance from each station is determined by keeping track of the time the earthquake waves arrived at each station.
Map out the positions of each seismograph station.
Draw circles with an equal radius around each seismograph station using the distance information. Each circle's radius is equal to how far away the station is from the epicenter.
At the place where the circles converge, there is an epicenter.
It is crucial to keep in mind that determining the epicenter of an earthquake is not an exact science, and the precision of the position will vary depending on a number of variables, such as the caliber of the seismograms and the distance between the earthquake and the seismograph stations.
To know more about epicenter, visit:
https://brainly.com/question/14261978
#SPJ9
Calculate the ph of a buffer solution that contains 0.25 M benzoic acid (c6h5co2h) and 0.15M sodium benzoate (c6h5coona) . Ka=6.5×10-5
Answers
Answer:
4.0
Explanation:
we have a buffer system formed by a weak acid (benzoic acid C₆H₅O₂H) and its conjugate base (benzoate ion C₆H₅O₂⁻, coming from sodium benzoate C₆H₅O₂Na). Given the acid dissociation constant (Ka) for benzoic acid is 6.5 × 10⁻⁵, we can calculate the pH of the buffer solution using the Henderson-Hasselbach equation.
pH =pKa + log [base]/[acid]
pH =-log Ka + log [C₆H₅O₂⁻]/[C₆H₅O₂H]
pH =-log 6.5 × 10⁻⁵ + log (0.15 M/0.25 M) = 4.0
Calculate the volume of 0.07216 M AgNO3 needed to react exactly with 0.3572 g of pure Na2CO3 to produce solid Ag2CO3.
Answers
Answer:
93.4 mL
Explanation:
Let's state the reaction:
2AgNO₃ + Na₂CO₃ → Ag₂CO₃ + 2NaNO₃
We determine the moles of sodium carbonate:
0.3572 g . 1mol / 105.98g = 3.37×10⁻³ moles
Ratio is 1:2. We say:
1 mol of sodium carbonate react to 2 moles of silver nitrate
Then, our 3.37×10⁻³ moles of carbonate may react to: 3.37×10⁻³ . 2
= 6.74×10⁻³ moles
If we convert to mmoles → 6.74×10⁻³ mol . 1000 mmol / mol = 6.74 mmol
Molarity is mol/L but we can use mmol /mL
6.74 mol / volume in mL = 0.07216 M
6.74 mol / 0.07216 M = volume in mL → 93.4 mL
What is the volume of 12.0 gof a liquid with a density of 1.37 g/mL?
Answers
c) Discuss precision and Accuracy as they relate to types of errors.
what is the answer
Answers
Precision relates to the consistency and reproducibility of measurements, while accuracy reflects how close measurements are to the true value.
Precision and accuracy are two important concepts in the context of errors in measurements. While they both pertain to the quality of data, they refer to different aspects.
Precision refers to the degree of consistency or reproducibility in a series of measurements. It reflects the scatter or spread of data points around the average value. If the measurements have low scatter and are tightly clustered, they are considered precise. On the other hand, if the measurements have a high scatter and are widely dispersed, they are considered imprecise.
Accuracy, on the other hand, refers to the closeness of measurements to the true or target value. It represents how well the measured values align with the actual value. Accuracy is achieved when measurements have a small systematic or constant error, which is the difference between the average measured value and the true value.
Errors in measurements can be classified into two types: random errors and systematic errors.
Random errors are associated with the inherent limitations of measurement instruments or fluctuations in the measurement process. They lead to imprecise data and affect the precision of measurements. Random errors can be reduced by repeating measurements and calculating the average to minimize the effect of individual errors.
Systematic errors, on the other hand, are caused by consistent biases or inaccuracies in the measurement process. They affect the accuracy of measurements and lead to a deviation from the true value. Systematic errors can arise from factors such as instrumental calibration issues, environmental conditions, or experimental techniques. These errors need to be identified and minimized to improve the accuracy of measurements.
In summary, precision refers to the degree of consistency or reproducibility of measurements, while accuracy refers to the closeness of measurements to the true value. Random errors affect precision, while systematic errors affect accuracy. To ensure high-quality measurements, both precision and accuracy need to be considered and appropriate techniques should be employed to minimize errors.
Know more about Precision here:
https://brainly.com/question/30461151
#SPJ8
the ideal constant has a value of 0.0821 as long as the units for the other variables are...

Answers
Answer:
Option D. atm, L, K, mole
Explanation:
To know which option is correct, do the following:
We shall use the standard value for each variable to obtain the gas constant. This can be obtained as follow:
Volume (V) = 22400 mL
Pressure (P) = 760 mmHg
Number of mole (n) = 1 mole
Temperature (T) = 273 K
Gas constant (R) =?
PV = nRT
R = PV / nT
R = (760 × 22400) / (1 × 273)
R = 62358.97 mmHg.mL/Kmol
Volume (V) = 22.4 L
Pressure (P) = 760 mmHg
Number of mole (n) = 1 mole
Temperature (T) = 273 K
Gas constant (R) =?
PV = nRT
R = PV / nT
R = (760 × 22.4) / (1 × 273)
R = 62.359 mmHg.L/Kmol
Volume (V) = 22400 mL
Pressure (P) = 1 atm
Number of mole (n) = 1 mole
Temperature (T) = 273 K
Gas constant (R) =?
PV = nRT
R = PV / nT
R = (1 × 22400) / (1 × 273)
R = 82.05 atm.mL/Kmol
Volume (V) = 22.4 L
Pressure (P) = 1 atm
Number of mole (n) = 1 mole
Temperature (T) = 273 K
Gas constant (R) =?
PV = nRT
R = PV / nT
R = (1 × 22.4) / (1 × 273)
R = 0.0821 atm.L/Kmol
From the above illustrations, we can see that the gas constant will have a value for 0.0821 as long as other variables are: atm, L, K, mole
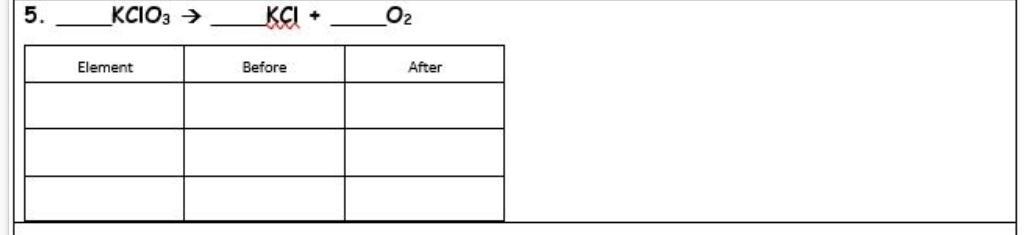
Balance Chemical Equations, will give brainiest.



Answers
*☆*――*☆*――*☆*――*☆*――*☆*――*☆*――*☆*――*☆**☆*――*☆*――*☆*――*☆
1) 2KCIO3=2kci+3o2
2)CH4 + 2O2 → CO2 + 2H2O
3) 2HCl + Ca(OH)2 → CaCl2 + 2H2O
I hope this helped!
<!> Brainliest is appreciated! <!>
- Zack Slocum
*☆*――*☆*――*☆*――*☆*――*☆*――*☆*――*☆*――*☆**☆*――*☆*――*☆*――*☆
Describe a process that may have produced the moon
Answers
Which of the following is a true statement?
All flat-topped hills are plateaus.
Volcanoes form all flat-topped hills.
All flat-topped hills are mesas.
Flat-topped hills can either be plateaus or mesas.
Answers
Answer:
D Flat Top Hills Can Be Plateaus or mesas
Explanation:
An object has a mass of 15.2 g and a volume of 8.9 mL.
What is the density of the object?
Answers
Answer:
recordando también que ** 1mL = 1cm ^ 3 **
(como ** 1 dm ^ 3 = 1 L **)
What mass (grams) of antimony(III) chloride would be produced by reacting with 112 liters of chlorine measured at STP?
Answers
Answer:
radius = 16 in ; height = 27 in
5. A reaction in which them material that starts the reaction is also one of the products and can start another reaction is called
a. An accelerator reaction
b. A moderator reaction
c. A shielding reaction
d. A chain reaction
Answers
Answer:
rhrhrhrhrhrhrrhrhrhrh
Explanation:
jrjrut5jt vrm VIP fo fo di xiu xiu cdi Deㅗ도돋ㅎㄷㄷㅎㄷㅎㄷㅍㄷuehehrgrvrrvhi UV co cu tu sew ccue
A gas has a temperature of 400. K, a pressure of 50.0 kPa, and a volume of 3.00 L. The volume is doubled while the temperature is held con- stant, and then the temperature is halved while the pressure is held constant. Use the gas laws to deter- mine the final temperature, pres- sure, and volume of the gas.
Answers
The, new volume is 1200 L and new pressure is 25 kPa.
Given, temperature = 400 K
Pressure = 50 kPa
Volume = 3 L
V1/T1 = V2/T2
V2 = V1×T2 / T1
V2 = 400× 6/ 2
V2 = 1200 L
Hence, new volume is 1200 L.
P1/T1 = P2/T2
P2 = P1 × T2/ T1
P2 = 50 × 1000 × 200/ 400
P2 = 25000 Pa
Hence, new pressure is 25 kPa.
A gas is a set of molecules that do not cohere strongly sufficient to form a liquid or a strong. The quantity of a gas is, so long as the molecules fit into it, the extent of the container preserving the gas. In different phrases, the extent of a gas isn't always a belongings of the gas immediately, however a belongings of the box.
Learn more about volume here:- https://brainly.com/question/25736513
#SPJ1
Which argument best explains the charge of an atomic nucleus? NEED HELP!!!!!!!
A. An atomic nucleus is positively charged because it is composed of neutrons.
B. An atomic nucleus is positively charged because it is composed of protons.
C. An atomic nucleus is negatively charged because it is composed of electrons.
D. An atomic nucleus is negatively charged because it is composed of neutrons.
Answers
Answer:
A. An atomic nucleus is positively charged because it is composed of neutrons.
Explanation:
What is the first step for response to an emergency situation?
Answers
Answer:
Back away from the situation and tell the supervisor/teacher
Why are scientific journals considered more reliable sources than web pages for information about science?
Web pages are too difficult to make.
Scientists never lie about their data.
Scientific journals are read and openly criticized by many scientists.
Web pages are subject to strict standards of evidence and sources.
Answers
Scientific journals considered more reliable sources than web pages for information about science, because, they are read and openly criticized by many scientists and publish only after expert committee revision.
What are scientific journals ?Scientific journals are books or magazines communicating the scientific inventions and discoveries conducted by a panel of scientists or research scholars through well designed experiments.
The communication of results is the last step in the research methodology. It is published in a journal only after the strict revision of the results and explanations by a panel of experts. Hence, no guidelines are violated here and it is not possible to publish an erroneous result.
In the case of web pages, it can be opened or edited by many users and and not all web pages are following the guidelines of communications. Therefore, scientific journals considered more reliable sources than web pages.
Find more on scientific journals:
https://brainly.com/question/567892
#SPJ1
Lab reaction rate project for chemistry edge2020

Answers
Answer:
What Affects Reaction Rate?
The purpose of this lab was to see how temperature and particle size affects reaction rate. The first hypothesis is if you increase the temperature of a reaction, then the reaction rate will increase because particles experience more collisions at higher temperatures.The second hypothesis is if you decrease the particle size of a reactant, then the reaction rate will increase because more of the reactants’ molecules will contact each other. The independent variables are particle size and temperature. The dependent variable is reaction rate.
Materials
250 mL graduated cylinder
Thermometer
Water
Timer
Four 250 mL beakers
Seven 1,000 mg effervescent tablets
Two pieces of filter paper
600 mL beaker
Ice
Hot plate
Procedure
Step 1:Gather Materials
Variation of Temperature
Step 2:Measure the Reaction Rate at ≈ 20°C (Room Temperature)
a) Using a graduated cylinder, fill a 250 mL beaker with 200 mL of water.
b) Measure the temperature of the water and record it in the correct row of Table A.
c) Reset the timer. Start the timer as you place a full tablet into the beaker.
d) Record the reaction time on the Data Sheet in the correct row of Table A.
e) Compute the reaction rate to the nearest mg/L/sec. Record it in the last column of Table A. Measure the Reaction Rate at ≈ 40°C
Step 3:Repeat Step 2, heating the water to approximately 40°C using a hot plate during sub-step a. Measure the Reaction Rate at ≈ 65°C
Step 4:Repeat Step 2, heating the water to approximately 65°C using a hot plate during sub-step a. Measure the Reaction Rate at ≈ 5°C
Step 5:Repeat Step 2, chilling the water to approximately 5°C inside an ice bath during sub-step a. (To create an ice bath, place 100 mL of ice and 100 mL of water in a 600 mL beaker of ice water and wait until the temperature reaches approximately 5°C. To save time, you may wish to set up the ice bath, using an additional 250 mL beaker, while working on Step 4.)
Variation of Particle Size
Step 6:Measure the Reaction Rate for a Full Tablet
a) Using a graduated cylinder, fill a 250 mL beaker with 200 mL of water.
b) Reset the timer. Start the timer as you place the tablet in the beaker.
c) Record the reaction time on the Data Sheet in the appropriate row of Table B.
d) Compute the reaction rate to the nearest mg/L/sec. Record it in the last column of Table B.
Step 7:Measure the Reaction Rate for a Partially Broken Tablet
Repeat Step 6, but this time break the tablet into eight small pieces on a piece of filter paper. Make sure to place all of the pieces into the beaker at the same time.
Step 8:Measure the Reaction Rate for a Crushed Tablet
Repeat Step 6, but this time crush the tablet into tiny pieces on a piece of filter paper. Make sure to place all of the pieces into the beaker at the same time.
Step 9: Dispose of all samples according to your teacher’s directions.
Measured Reaction Temperature (°C)
Mass of Tablet (mg)
Volume of Water (L)
Reaction Time (s)
Reaction Rate (mg/L/s)
≈20°C
24
1,000
0.2
34.2
146.2
≈40°C
40
1,000
0.2
26.3
190.1
≈65°C
65
1,000
0.2
14.2
352.1
≈5°C
3
1,000
0.2
138.5
36.1
Relative Particle Size (Small, Medium, Large)
Mass of Tablet (mg)
Volume of Water (L)
Reaction Time (s)
Reaction Rate (mg/L/s)
Full Tablet
large
1,000
0.2
34.5
144.9
Broken Tablet
medium
1,000
0.2
28.9
173.0
Crushed Tablet
small
1,000
0.2
23.1
216.5
The data in the first table show that as the temperature increases the reaction time decreases and in turn the reaction rate increases. The data supported the hypothesis that as temperature increases reaction rate will also increase. The second table shows that as the particle size decreases the reaction time increases because there is more surface area when the particles are smaller. The data in the second table supported the second hypothesis that as particle size decreases the reaction rate will increase because there will be more contact in the molecules. Possible source of error would be an error in stopping the timer in time or chips in the tablets. To improve this lab it could be done with different types of reactions or different temperature or different particle sizes.
Explanation:
Answer:
The person above is amazing and gave us all the answers!!
Explanation:
A classroom has one triple-beam balance. To complete an experiment, each student must use the balance for 6 minutes. The class meets for 1 hour. How many students can participate in the experiment?
Answers
As the class is of 1 hour and and each student uses for 6 minutes so in 1 hour 10 students will use the balance.
What is a balance?
A scale or balance is a device used to measure weight or mass. These are also known as mass scales, weight scales, mass balances, and weight balances.
The traditional scale consists of two plates or bowls suspended at equal distances from a fulcrum. One plate holds an object of unknown mass (or weight), while known masses are added to the other plate until static equilibrium is achieved and the plates level off, which happens when the masses on the two plates are equal.
Learn more about balance,here:
https://brainly.com/question/1173599
#SPJ1
A sample consisting of 65.0 g of Xenon is confined in a container at 2.0 atm and 298 K. The gas (assume ideal gas behaviour) is expanded adiabatically and reversibly to a final pressure of 1.0 atm. Calculate q, ∆T, w, ∆U, and ∆H.
Answers
The values of q = 0, ΔT = 50K, w= ΔU = 509.23J.
What is Adiabatic expansion?
Adiabatic expansion is defined as the expansion in which there is no heat interaction of the system with the surroundings and work is done by the system at the expense of its internal energy.
Given,
Mass of Xe = 65g
Initial Pressure = 2 atm
T = 298K
Final pressure = 1 atm
Moles of Xe = mass ÷ molar mass
= 65 ÷ 131 = 0.49 moles
Since it is adiabatic, q = 0
Δ U = w
Cv = 5/2 R
nCv (T₂ - T₁) = \(\frac{nRT_{2} }{P_{2} }\) - \(\frac{nRT_{1} }{P_{1} }\)
Substituting the values as above, we get,
T₂ = 249 K
ΔT = 298 - 248 = 50K
ΔU = w = n Cv ΔT
= 0.49 × 20. 78 × 50
= 509.23 J
Therefore, the values of q = 0, ΔT = 50K, w= ΔU = 509.23J.
Learn more about Adiabatic Expansion, here:
https://brainly.com/question/4597803
#SPJ1
Which of the following observations about burning sugar provides evidence of a
chemical reaction?
a. Heat is added to the sugar crystals.
b. The sugar melts and becomes a liquid.
с
C. The temperature of the sugar increases.
d. Gas is produced as the sugar turns black.
1 Pt
А
B
D
Answers
How many moles of tin, Sn, are in 2500 atoms of tin?
Answers
4.2 × 10⁻²¹ moles Sn
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Using Dimensional AnalysisAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Explanation:Step 1: Define
2500 atoms Sn
Step 2: Identify Conversions
Avogadro's Number
Step 3: Convert
Set up: \(\displaystyle 2500 \ atoms \ Sn (\frac{1 \ mol \ Sn}{6.022 \cdot 10^{23} \ atoms \ Sn})\)Multiply: \(\displaystyle 4.15144 \cdot 10^{-21} \ moles \ Sn\)Step 4: Check
Follow sig fig rules and round. We are given 2 sig figs.
4.15144 × 10⁻²¹ moles Sn ≈ 4.2 × 10⁻²¹ moles Sn
The heart is an organ in the circulatory system. Muscle tissue in the heart contracts to pump blood to the body. Connective and epithelial tissues in the heart hold the muscle cells together and in place in the chest. Nervous tissue in the heart coordinates how fast and hard the muscle cells contract.
Based on the information about the heart, which of these best describes the relationship between tissues and organs?
Answers
The relationship between tissues and organs is one of interdependence. Tissues work together to form organs, and organs rely on the different types of tissues to function properly.
The relationship between tissues and organs is an important one, and it is particularly exemplified in the case of the heart. The heart is an organ, and like all organs, it is made up of various types of tissues that work together to allow it to function properly. In the case of the heart, these tissues include muscle tissue, connective tissue, epithelial tissue, and nervous tissue.
Muscle tissue is particularly important in the heart, as it contracts to pump blood to the body. Without muscle tissue, the heart would not be able to perform its vital function. Connective and epithelial tissues are also important in the heart, as they hold the muscle cells together and in place in the chest. Without these tissues, the muscle cells would not be able to work together efficiently, and the heart would not be able to function properly.
Finally, nervous tissue plays a crucial role in the heart, as it coordinates how fast and hard the muscle cells contract. This coordination is essential for the heart to function properly and maintain the circulation of blood throughout the body. In the case of the heart, the different types of tissues work together seamlessly to allow the heart to perform its vital function of pumping blood throughout the body.
For such more questions on organs
https://brainly.com/question/545314
#SPJ11
Answer: A
Explanation:
I got C wrong
What is it called when an electron is in the lowest possible energy level?Schrodinger stateExcited stateBohr stateGround state
Answers
ANSWER
Ground state
EXPLANATION;
Ground-state atom is an atom in which the total energy of the electrons cannot be lowered by transferring one or more electrons to different orbitals.
This implies that, in the ground-state atom all electrons are in their lowest possible energy level.
Hence, the correct answer is ground state.
what's element on the periodic table has the symbol w
Answers
Answer:
W is tungsten on the periodic table