Answers
Answer:
Only plant cells
Explanation:
This organelle helps plants photosynthesize. Humans do not do that.
Related Questions
whats the energy in joules of one mole of photons of visible light having a wavelength of 4.11×10^2 nm?
Answers
The energy in joules of one mole of photons of visible light having a wavelength of 4.11×10^2 nm? can be expressed as 2.9*10^5 J
How can the energy be calculated?From the question we were told to find the energy and the parameters that is needed to calculate these are;
c=3*10^8
h= 6.626 * 10^-34 J.s
1 mol photons = 6.023x10^23 photon
λ = 4.11×10^2 nm = 4.11 × 10-7 meters
The parameters can be input as Energy of one mole photon (E) = ( 6.023x10^23 * 6.626 * 10^-34 * 3*10^8)/ (4.11 × 10^-7)
=291302
=2.9*10^5 J
Learn more about energy at:
https://brainly.com/question/13881533
#SPJ1
Hydrogen reacts with oxygen according to the balanced equation
2H₂ (g) + O2(g) → 2H₂O(g). If X is the number of molecules of H₂ which react,
then the number of O2 molecules reacting is
Answers
Answer:
x/2
Explanation:
X = 2 molecules of H2
For 2 molecules of H2, there's only 1 molecule of O2. Meaning, there's twice the amount of H2, so O2 = x/2 molecules.
I hope I'm understanding this question right.
QUESTION 2
Prepare 25 mL of a 60 mg/mL solution from a stock supply of 0.5 g/5 mL.
How many milliliters of the solute (stock solution) are needed?
How many milliliters of the solvent (water) are needed?
O a. 15 mL solute; 10 mL solvent
O b. 21 mL solute; 8 mL solvent
O c. 17 mL solute; 9 mL solvent
O d. 25 mL solute; 10 mL solvent
Answers
The number of solute (stock solution) needed are 15 mL solute and the number of solvents (water) is needed 10 ml. The correct option is a.
What is molarity?Molarity is the measure of the concentration of any solute per unit volume of the solution.
Given that the 25 mL of a 60 mg/mL solution
Dose in hand = 0.5 g / 5ml =
500 mg / 5ml = 100 mg/ml
D= 60 mg x 25 = 1500 mg
Molarity = n / V
1500 mg / 100 mg/ml = 15 ml solute
The number of solutes then minus by the total stock supply
15 - 5 ml = 10 ml solvent
Thus, the correct option is a. 15 mL solute; 10 mL solvent.
To learn more about molarity, refer to the link:
https://brainly.com/question/14562993
#SPJ1
2.92 A 50.0-g silver object and a 50.0-g gold object are both added
to 75.5 mL of water contained in a graduated cylinder. What is
the new water level in the cylinder? (2.7)
ifacturing of computer chins cylinders of silicon
Answers
Answer:
82.9 mL
Explanation:
1. Volume of silver
\(\begin{array}{rcl}\text{Density}&=& \dfrac{\text{Mass}}{\text{Volume}}\\\\\rho&=& \dfrac{m}{V}\\\\V &=& \dfrac{m}{\rho}\\\\& = & \dfrac{\text{50.0 g}}{\text{10.49 g$\cdot$mL}^{-1}}\\\\& = & \text{4.766 mL}\\\end{array}\\\text{The volume of the silver is $\large \boxed{\textbf{4.766 mL}}$}\)
2. Volume of gold
\(\begin{array}{rcl}V& = & \dfrac{\text{50.0 g}}{\text{19.30 g$\cdot$mL}^{-1}}\\\\& = & \text{2.591 mL}\\\end{array}\\\text{The volume of the gold is $\large \boxed{\textbf{2.591 mL}}$}\)
3. Total volume of silver and gold
V = 4.766 mL + 2.591 mL = 7.36 mL
4 New reading of water level
V = 75.5 mL + 7.36 mL = 82.9 mL
The photo shows a Newton's cradle. The ball on the left can be pulled away
from the others. After the ball is released, it strikes the next ball in line and
the ball on the far right side of the toy moves up and away,
What most likely explains the motion of the ball on the far right?
A. The mechanical energy of the ball on the left is transferred in the
form of waves to the ball on the right
B. The potential energy of the ball on the left is transferred directly to
the ball on the right
C. The potential energy of the ball on the left is transferred in the
form of waves to the ball on the root
D. The mechanical energy of the ball on the left is transferred through
the other balls to the ball on the right

Answers
The characteristics of the conservation of energy and momentum allow to find the result for the movement of the balls, the correct answer is:
D. The mechanical energy of the ball on the left is transferred through
the other balls to the ball on the right.
Mechanical energy is the sum of the kinetic energy and the potential energies. In the case that there is no friction, the mechanical energy is conserved.
The momentum is defined by the product of the mass and the velocity of the body, in an isolated system the momentum is conserved and the shocks can be of two types:
Inelastic. Where part of the energy is transformed into internal energy and the kinetic energy is not conserved. Elastic. Where kinetic energy is conserved.
In the case indicated, when removing the ball, it gains a height, therefore if the initial mechanical energy is
Em₀ = mgh
When it reaches the lowest point, just before it hits the ball its energy is:
\(Em_f\) = 1 / 2m v²
Em₀ = Em_f
v = \(\sqrt{2gh}\)
collides with the other ball and transfers its momentum in an elastic collision, the balls do not stick, as all the energy of the ball is kinetic, this energy is transferred to each ball until it reaches the last one, where the kinetic energy is transformed into potential energy a increases its height.
Let's analyze the answers:
A. False. This is a collision of particles there are no waves.
B. False. At the lowest point the ball on the left has kinetic energy.
C. False. It is a collision of particles, there are no waves.
D. True. The energy from the elastic collisions is transferred through each ball until it reaches the last ball.
In conclusion, using the characteristics of the conservation of energy and momentum, we can find the result for the movement of the balls, the correct answer is:
D. The mechanical energy of the ball on the left is transferred through the other balls to the ball on the right
Learn more here: brainly.com/question/3920210
A sample of oxygen is subjected to an absolute pressure of 2.4 atm. If the specific internal energy of the sample at 310 K is 5700 J/mol relative to a known reference state, what is the specific enthalpy of the oxygen relative to that same reference state?
Answers
Answer:
The required specific enthalpy for oxygen = 8277.34 J/mol
Explanation:
Given that :
Pressure = 2.4 atm
Temperature = 310 K
specific internal energy U = 5700 J/mol
To find the specific enthalpy using the formula:
H = U + PV
where;
H is known as the specific enthalpy
Recall that from ideal gas equation
PV = nRT
For specific enthalpy, it is constant that n = 1
Thus;
PV = RT
replacing that into the equation (H = U + PV), we have:
here;
R = 8.314 J/mol K (constant)
H = U + RT
H = 5700 J/mol +( 8.314 J/mol K × 310 K)
H = 5700 J/mol + ( 2577.34 J/mol)
H = 8277.34 J/mol
do mixtures boil at a constant temperature
Answers
Answer:
no
Explanation:
different mixtures have different boiling and melting points
Answer:
Yes, they do.
Explanation:
To boil at a constant temperature, the vapour composition of the mixture has to account for liquid mixture composition.
And these mixtures are known as azeotropes or azeotropic mixture
\({}\)
Write condensed and bond-line structural formulas for all of the constitutional isomers Practice problem 4.1 with the molecular formula C7H16. (There are a total of nine constitutional isomers.)
Answers
Answer:
See figure 1.
Explanation:
In this case have to take into account that all structures must have the formula: \(C_7H_16\). If we remember the general formula for alkanes: \(C_nH_2_n_+_2\) if we have "7" carbons (n=7) we will have 16 hydrogens. Therefore all the structures that fit with this formula are alkanes.

The solvent for an organic reaction is prepared by mixing 70.0 mL of acetone (C3H6O) with 75.0 mL of ethyl acetate (C4H8O2). This mixture is stored at 25.0 ∘C. The vapor pressure and the densities for the two pure components at 25.0 ∘C are given in the following table. What is the vapor pressure of the stored mixture?
Answers
Answer:
The answer is "170.9 mm Hg".
Explanation:
\(\text{Mass of acetone = volume} \times density\)
\(= 70.0 \times 0.791\\\\ = 55.37\ g\\\)
\(\text{Moles of acetone} = \frac{mass}{molar\ mass}\\\\\)
\(=\frac{55.37}{58.08}\\\\ = 0.9533\ mol\)
\(\text{Mass of ethyl acetate = volume} \times density\)
\(= 73.0 \times 0.900\\\\ = 65.7\ g\)
\(\text{Moles of ethyl acetate = mass} \times\ molar\ mass\)
\(= \frac{65.7}{88.105} \\\\= 0.7457\ mol\)
\(\text{Mole fraction of acetone x(acetone)} = \frac{0.9533}{(0.9533 + 0.7457)}\ = 0.5611\\\\\) \(\text{Mole fraction of ethyl acetate x(ethyl acetate)} =\frac{0.7457}{(0.9533 + 0.7457) }= 0.4389\)
Applying Raoult's law: \(\text{Vapor pressure = x(acetone)P(acetone) + x(ethyl acetate)P(ethyl acetate)}\\\\= 0.5611 \times 230.0 + 0.4389 \times 95.38\\\\ = 170.9\ mm \ Hg\\\)
The solvent for an organic reaction is prepared by mixing 70.0 mL of acetone (C3H6O) with 75.0 mL of ethyl acetate (C4H8O2).
The vapor pressure of the stored mixture is: 170.03 mmHg
In the given information, there is some information that is still missing.
The parameters that we are being given include:
The volume of acetone = 70.0 mLThe volume of ethyl acetate = 75.0 mLThe standard temperature for the mixture = 25° CThe first step we need to take is to determine the mass and number of moles of each compound (i.e. for acetone and ethyl acetate)
For us to do that:
We need the density of acetone and ethyl acetate, which is not given:
Assuming that at a standard condition of vapour pressure:
230 mmHg of acetone has a density of 0.791 g/mL95.38 mmHg of ethyl acetate has a density of 0.900 g/mLThen;
Using the relation:
\(\mathbf{Density = \dfrac{Mass}{volume}}\)
Mass of acetone = Density of acetone × volume of acetone
Mass of acetone = 0.791 g/mL × 70.0 mL
Mass of acetone = 55.37 g
Mass of ethyl acetate = Density of ethyl acetate × volume of ethyl acetate
Mass of ethyl acetate = 0.900 g/mL × 75.0 mL
Mass of ethyl acetate = 67.5 g
At standard conditions;
For acetone, molar mass = 58.08 g/molFor ethyl acetate, molar mass = 88.11 g/molNow, using the formula for calculating the numbers of moles which can be expressed as:
\(\mathbf{Number \ of \ moles = \dfrac{mass}{molar \ mass}}\)
For acetone:
\(\mathbf{Number \ of \ moles = \dfrac{55.37 \ g}{58.08 \ g/mol}}\)
\(\mathbf{Number \ of \ moles =0.95334 \ mol}\)
For ethyl acetate:
\(\mathbf{Number \ of \ moles = \dfrac{67.5 \ g}{88.11 \ g/mol}}\)
\(\mathbf{Number \ of \ moles =0.76609 \ mol}\)
Now, we will determine the mole fraction of each compound.
The mole fraction describes the ratio a certain constituent of a mixture to the total amount of all the constitutent in the mixture.
Using the formula:
\(\mathbf{mole \ fraction = \dfrac{n_A}{n_A+n_B+...n_N}}\)
For Acetone:
\(\mathbf{mole \ fraction = \dfrac{0.95334}{0.95334+0.76609}}\)
\(\mathbf{mole \ fraction =0.5545 }\)
For ethyl acetate:
\(\mathbf{mole \ fraction = \dfrac{0.76609}{0.76609+0.95334}}\)
\(\mathbf{mole \ fraction =0.4455}\)
Finally, we can compute determine the vapour pressure of the stored mixture using Raoult's Law.
Raoult's Law posits that the constituent of a partial pressure in a mixture of a liquid is proportional to the mole fraction of that constituent in the mixture provided the temperature is constant.
∴ For the stored mixture = Vapor pressure of acetone + vapour pressure of ethyl acetate.
where:
Vapour pressure of the solution = (mole fraction × vapor pressure) of solventFor acetone;
Vapor pressure = 0.5545 × 230 mmHg
Vapour pressure = 127.54 mmHg
For ethyl acetate:
Vapour pressure = 0.4455 × 95.38 mmHg
Vapour pressure ==42.49 mmHg
Thus, the vapor pressure of the stored mixture is
= (127.54 + 42.49 ) mmHg
= 170.03 mmHg
Therefore, we can conclude that the vapour pressure of the stored mixture is 170.03 mmHg
Learn more about Vapour pressure here:
https://brainly.com/question/16931217?referrer=searchResults
What coefficient of 02 should be added so the number of atoms of oxygen is conserved on both sides of the reaction equation
Answers
Answer:
5
Explanation:
Which expression represents the concentration of OH– in solution?
a. 10–14 / [H3O+]
b. [OH–] / 10–14
c. 10–14 – [H3O+]
d. 10–14 x [H3O+]
Answers
Explanation:
Hydronium (H3O+) and hydroxide ions (OH-) are both present in pure water and in all aqueous solutions.
Their respective concentrations in water are 10^-7 M each and are inversely proportional to each other as given by the ion product of water, Kw.
Kw = [H3O+][OH−]
Where Kw = 1.0 * 10^-14,
[H3O+] = concentration of hydronium ions
[OH-] = concentration of hydroxide ions
Therefore, [OH-] = Kw / [H3O+]
[OH-] = 10^-14/[H3O+]
Which metal does not form cations of differing charges?
Answers
Transition metals
Most transition metals differ from the metals of Groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. As an example, iron commonly forms two different ions
Which object represents a heterogeneous
mixture
Answers
Answer:
oil and water
Explanation:
oil and water - When you put oil and water together they do not mix. Therefore, these are a heterogeneous mixture since they are two separate parts.
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
A chemical adds 1.10 L of a 0.384mol/L barium chloride
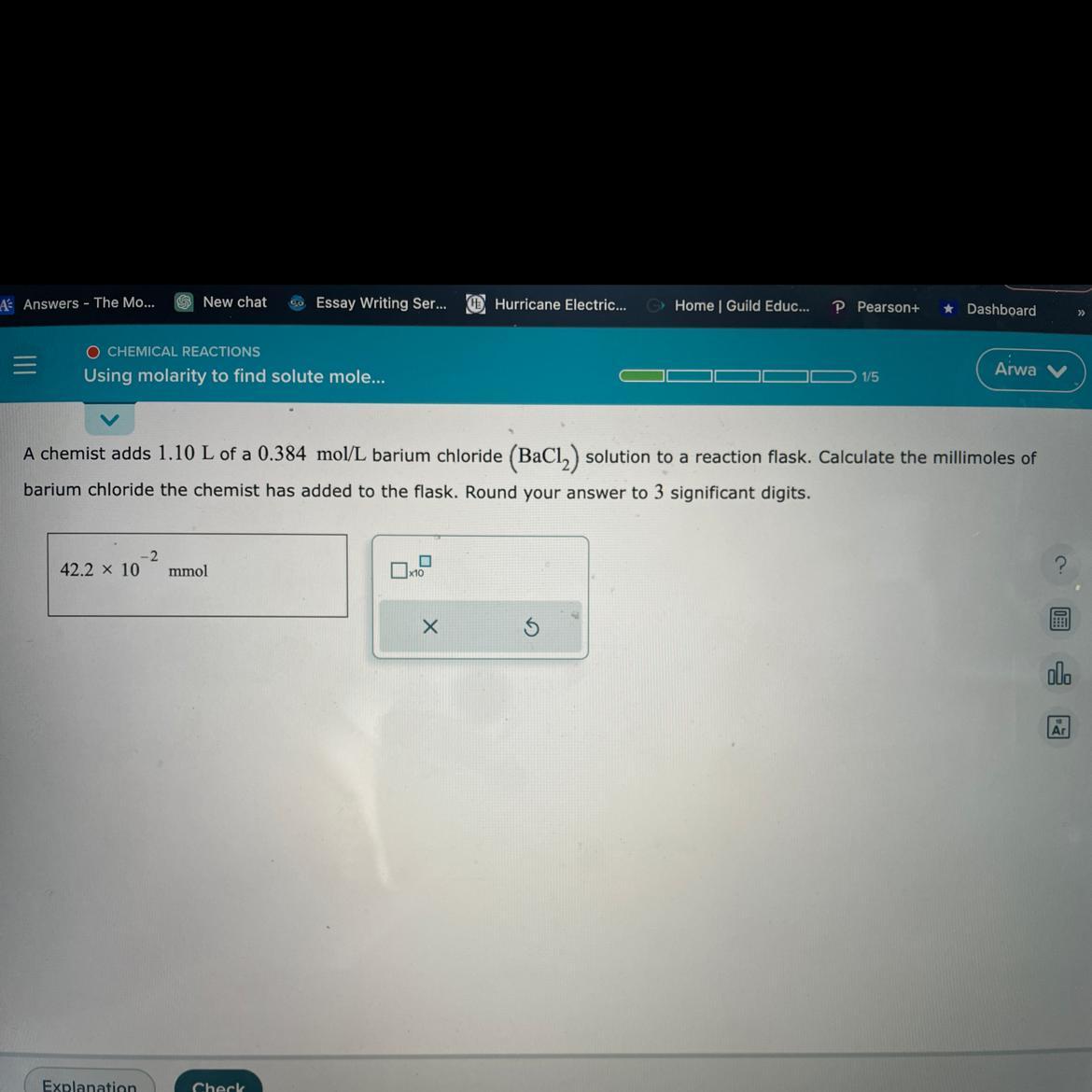
Answers
The number of millimoles of barium chloride added to the flask to 3 significant figures would be 422 mmol.
Number of moles calculationTo calculate the millimoles of barium chloride added to the flask, we need to use the following formula:
moles = concentration x volume
where concentration is in units of mol/L, and volume is in units of L. We are given that the volume is 1.10 L and the concentration is 0.384 mol/L, so:
moles = 0.384 mol/L x 1.10 L
moles = 0.4224 mol
Now, to convert moles to millimoles, we multiply by 1000:
millimoles = 0.4224 mol x 1000
millimoles = 422.4 mmol
Therefore, the millimoles of barium chloride added to the flask is 422 mmol (rounded to 3 significant figures).
More on number of moles can be found here: https://brainly.com/question/12513822
#SPJ1
Other than the starting material, 2-methylcyclohexanol, what base or bases are present in the dehydration reaction mixture to participate in proton transfers?
a) H2PO4- only
b) H2O and H2PO4-
c) H2O and HSO4-
d) H2O only
Answers
Answer:
c) H2O and HSO4-
Explanation:
The dehydration of 2-methylcyclohexanol occurs in the presence of H2SO4. Recall that acids are proton donors in solution; the equilibrium shown below is then set up,
H2SO4(aq) + H2O(l) ⇄H3O^+(aq) + HSO4^-(aq)
The species H2O and HSO4- are two proton acceptors in the system. According to the Brownstead- Lowry definition, a base is a proton acceptor in solution.
Hence H2O and HSO4- are bases present in the dehydration of 2-methylcyclohexanol.
1. Describe a situation in which you might need to convert the units of a measurement, and
what information you would need to do so.
Answers
A certain substance, X, decomposes. 75 % of X remains after 100 minutes. How much X remains after 200 minutes if the reaction order with respect to X is the following order: zero order, first order and second order.
Answers
At the equivalence point of a titration of the [H+] concentration is equal to:
Group of answer choices
A. 1 x 107 M
B. 7
C. [OH-]
D. 1 x 10-7 M
Answers
B. At the equivalence point of a titration of the [H+] concentration is equal to 7.
What is equivalence point of a titration?The equivalence point of a titration is a point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution.
At the equivalence point in an acid-base titration, moles of base equals moles of acid and the solution only contains salt and water.
At the equivalence point, equal amounts of H+ and OH- ions combines as shown below;
H⁺ + OH⁻ → H₂O
The pH of resulting solution is 7.0 (neutral).
Thus, the pH at the equivalence point for this titration will always be 7.0.
Learn more about equivalence point here: https://brainly.com/question/23502649
#SPJ1
Using the reading "Fossil Fuels" from lesson 12 describe the environmental and economic benefits and drawbacks of fossil fuels. Second, looking over the benefits and drawbacks, in your opinion, what do you think will happen to mining of fossil fuels in the next 50 years?
Answers
Fossil fuels are essential part of the power generation in the world. They are easily combustible and more reliable and cheaper. However, the burning of fossil fuels releases toxic gases to the environment.
What are fossil fuels ?Fossil fuels are fuel generated from the decomposition materials. Petroleum, coal, natural gas etc. are fossil fuels which are excavating from the earth.
Fossil fuels are non-renewable sources of energy. Hence, as the existing fossil sources are exhausted no more fossil fuel can be made. It is cheaper, reliable and easy to use.
However, the toxic hydrocarbon gases released from the burning of fossil fuels make the environment polluted. Therefore, overuse of fossil fuel definitely rise the atmospheric pollution.
Its use over the next 50 years, will increase the global warming and more of it will be exhausted.
Find more on fossil fuels :
https://brainly.com/question/3371055
#SPJ1
In one combination of acid and amine, a white ring was observed almost exactly halfway between the two ends. Which acid and which amine were used
Answers
A combination of acid and amine with similar molecular masses would be used.
When two substances capable of diffusing are placed at extreme ends of a sealed tube, each will diffuse towards the opposite end of the tube.
If the 2 substances are capable of reacting to form a white ring like in the case of the acid and the amine, the point at which the white ring will form would depend on the rate of diffusion of each substance.
According to Graham's law of diffusion, the rate of diffusion of substances is inversely proportional to the square root of their molecular masses. Going by this law, gases with similar molecular weight will have similar rates of diffusion.
Thus, 2 substances (gases) with similar molecular masses, left to diffuse in the opposing ends of a sealed tube will diffuse to meet around the halfway point between the two ends of the tube where they can react and form a white ring.
More on diffusion rates can be found here: https://brainly.com/question/24746577?referrer=searchResults
Use the data in the table below to answer the following questions. The true value for this data set is 5.68g.

Answers
1) The student which is neither precise nor accurate is student B
2) The student which is most accurate on any single trial is student A
3) The student that is both precise and accurate is student C
4) The student which is precise but not accurate is student D
What is precision and accuracy?The term precision has to do with the fact that the values of the measurement are close together. Precise values do not scatter a lot. They vary within a narrow range.
An accurate measurement is one that is close to the true value. This means that it does not differ so much from the true value of the measurement.
Hence;
1) The student which is neither precise nor accurate is student B
2) The student which is most accurate on any single trial is student A
3) The student that is both precise and accurate is student C
4) The student which is precise but not accurate is student D
Learn more about precision and accuracy:https://brainly.com/question/15276983
#SPJ1
A sample of the compound weighs 80 grams. How many grams of cobalt are in the sample?
Answers
The mass of the unknown element cobalt is obtained as 40 g. This can be seen from the calculation that we have in the solution.
How can you use the molar mass of the compound to find the relative atomic mass of the unknown element?If you have a compound that contains an unknown element, you can use the molar mass of the compound to find the relative atomic mass of the unknown element
We can see that the question has already given us the mass of the sample as we have and the percentage of the cobalt that we have in the sample.
50 = x/80 × 100
x = 50/100 × 80
= 40 g
Thus we would have a total of about 40 g of cobalt in the sample.
Learn more about molar mass:https://brainly.com/question/22997914
#SPJ1
Missing parts;
A sample of the compound weighs 80 grams. If the mass percent of cobalt is 50%, How many grams of cobalt are in the sample?
Zootopia movie:
How does Judy Hopps discover Nick Wolf’s true intentions/con?
Answers
Answer:
Judy is determined to make the world a better place while breaking preconceptions about other species. Nick views Judy as a nuisance at first. He also mentions having noticed her canister of Fox repellent when they first met, leading him to believe her to be a bigoted individual, which he detests.
Explanation:
The Croatian seismologist, Andrija Mohorovicic, discovered a boundary change in the Earth's layers. Please explain how he discovered this using two to three sentences using your best grammar.
Answers
The Croatian seismologist, Andrija Mohorovicic, discovered a boundary change in the Earth's layers because he noticed that P-waves were refracted at the boundary.
Who is a seismologist?Seismologists are described as Earth scientists, specialized in geophysics, who study the genesis and the propagation of seismic waves in geological materials.
Andrija Mohorovicic realized that as one crosses the boundary the predominant mineral composition of the rock changes, and the minerals on the mantle side enable seismic waves to travel faster.
Learn more about Seismologists at: https://brainly.com/question/676287
#SPJ1
What is the new molarity if 55.0mL of water is added 25.0mL of 0.119M
NaCl solution.
Answers
Answer: 0.04M.
Explanation: Using M1V1=M2V2, we can find the new molarity (M2).
We are given M1 and V1 with 0.119M and 25mL, we are also given V2 since it says we added 55mL to the original 25mL.
25mL+55mL = 80mL.
Let's convert mL to L by dividing by 1000.
V1 = 25ml/1000 = 0.025L
V2 = 80mL/1000 = 0.08L.
Now plug in and solve algebraically.
0.119 * 0.025 = M2 * 0.08. Divide both sides by 0.08.
\(\frac{0.119*0.025}{0.08}\) = 0.037.
M2 = 0.037M
However, since we are given 25mL which only has 2 sig figs, the new molarity os 0.04M.
How many atoms are in 3.2 moles of S (sulfur)?
Answers
Answer:
1.92704e24 atoms
Explanation:
Using Avogadro's number, you can set up a stoichiometric equation to find the number of atoms.

There are 1.926 × 10²⁴ atoms in 3.2 moles of sulfur (S).
How to calculate number of atoms?The number of atoms in a substance can be calculated by multiplying the number of moles by Avogadro's number.
Avogadro's number is the number of atoms present in 12 grams of isotopically pure carbon-12, being 6.02214076 × 10²³.
According to this question, there are 3.2 moles of sulfur. The number of atoms can be calculated as follows:
no of atoms = 6.02 × 10²³ × 3.2
no of atoms = 19.26 × 10²³
no of atoms = 1.926 × 10²⁴
Therefore, 1.926 × 10²⁴ atoms are in 3.2 moles of sulfur.
Learn more about number of atoms at: https://brainly.com/question/8834373
#SPJ1
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
2.How might the structure of molecules help scientists determine how they interact with other molecules?
Answers
According to the molecular geometry, with the help of structure of molecules which provide information on site of attachment with other molecules, one can determine their mode of reaction.
What is molecular geometry?Molecular geometry can be defined as a three -dimensional arrangement of atoms which constitute the molecule.It includes parameters like bond length,bond angle and torsional angles.
It influences many properties of molecules like reactivity,polarity color,magnetism .The molecular geometry can be determined by various spectroscopic methods and diffraction methods , some of which are infrared,microwave and Raman spectroscopy.
Learn more about molecular geometry,here:
https://brainly.com/question/7558603
#SPJ1
Answer: The shape of a molecule helps to determine its properties, which affect how a molecule interacts with other molecules, such as polarity and bonding.
Much of earth surface is covered by groundwater, body of fresh water, land areas, or bodies of salt water?