Carbon 12, 13, and 14 are isotopes of the same element. They share the same place on the periodic table but possess varying numbers of neutrons. If carbon 12 has 6 protons, how many neutrons will carbon 14 have?.
Answers
Carbon 14 has 8 neutrons if carbon 12 has 6 protons.
Carbon 12, Carbon 13, and Carbon 14 are isotopes of the same element carbon which share the same place on the periodic table. Isotopes are atoms that have the same number of protons but different numbers of neutrons. The mass of an atom depends on the number of protons and neutrons in the nucleus.
The number of neutrons in an atom can be determined by subtracting the number of protons from the mass number. Carbon 12 has 6 protons and 6 neutrons, Carbon 13 has 6 protons and 7 neutrons, while Carbon 14 has 6 protons and 8 neutrons. Therefore, if Carbon 12 has 6 protons, Carbon 14, which is also an isotope of Carbon, will have 8 neutrons.
Learn more about isotopes here:
https://brainly.com/question/27475737
#SPJ11
Related Questions
X has a fixed melting and boiling point while Y does not. Which of the following statements about X and Y is correct?
A: X is an element or a compound while Y is a mixture.
B: X is an element while Y is a compound or a mixture.
C: X is a mixture while Y is an element or a compound.
D: X is a compound while Y is an element or a mixture.
Answers
Answer:
A. X is an element or a compound while Y is a mixture
Sometimes a fossil is formed as a result of the movement of an organism in soft sediment. What type of fossil is this called? bone fossil trace fossil frozen fossil petrified fossil
Answers
Answer:
The most common method of fossilization is called permineralization, or petrification.
Answer:
Trace Fossil
Explanation:
typically formed when an organism moves over the surface of soft sediment and leaves an impression of its movement behind.
Select the single replacement reaction.
A. Ca(OH)2 + CaO+H20
B. Zn + 2HCl → ZnCl2 + H2
C
H2SO4 + 2NaOH
Na2SO4 + 2H2O
D. 6CO2 + 6H2O → C6H12O6 + 602
Answers
The single replacement reaction in this question is: Zn + 2HCl → ZnCl2 + H2.
WHAT IS A SINGLE REPLACEMENT REACTION?A single replacement reaction is the type of reaction in which one element in a reaction is replaced by another element.
According to this question, the reaction given below is an example of single replacement reaction:
Zn + 2HCl → ZnCl2 + H2This is because hydrogen in HCl is replaced by Zinc element to form zinc chloride.
Learn more about single replacement reaction at: https://brainly.com/question/8625202
Rose petals contain a variety of different coloured pigments. A student wants to identify these pigments. Explain why
Answers
The petal of rose has different kinds of pigments like anthocyanins, carotenoids which give them the colors.
What are Anthocyanins?
Anthocyanins are the pigment that produces red color in the roses. They belong to Flavonoids family.
The pigments in a rose's petals give it its color. These pigments mostly consist of carotenoids and anthocyanins. Bright yellow, orange, and red pigments like those in lemons, oranges, and tomatoes are made by carotenoids. They are contained in structures called plastids, which are a component of the cytoplasm of plant cells. They are therefore steady and largely unaffected by the environment or the health of the plant. Deep red, magenta, purple, and blue colors are made of anthocyanins. They are transported via the sap of the plant and are water soluble. They are far less stable and more susceptible to external variables because they are in a fluid environment.
According to research, a bloom's anthocyanin content and sap pH fluctuate as a flower develops from a bud to a bloom. The pH of the sap increases with bloom aging and anthocyanin content decreases. As those who grow hydrangeas are aware, more acidic conditions prefer the pink/red end of the color spectrum while high alkaline levels produce blue colorations. As a result, as your rose bloom ages and its pH rises, the red will tend to lean toward the darker, bluer side of the spectrum. When a flower is in bloom, anthocyanin levels are still high, but they start to drop off swiftly as the blossom fades.
To know more about anthocyanin, click the following link
https://brainly.com/question/15609513
#SPJ9
If we reduce the volume in the vessel and the new pressure is measured to be 40 atm, what would the new volume be? a. 10 Lb. 5 Lc. 3.33 Ld. 2.5 L
Answers
At a pressure of 40 atm, the gas's new volume will be 2.5 L. As a result, choice (D) is the right one.
Describe Boyle's law.
According to Boyle's law, the pressure a given mass of gas exerts at a given temperature is inversely proportional to the volume that it occupies.
If the temperature remains constant, the relationship between the pressure and volume is inverse.
P ∝ 1/V
or
P₁V₁ = P₂V₂ ................(1) (1)
Given that the gas's starting pressure is P1 = 10 atm
The gas's ultimate pressure, P2, is 40 atm.
The gas's initial volume, V1, is equal to 10 L.
Replace the volume and pressure numbers in equation (1);
(40 atm) V2 = (10 atm) (10 L)
V₂ = 100/4
V₂ = 2.5 L
Consequently, the gas's new volume will be 2.5 L. 40 atm of pressure is increased,
For such more question on Boyle's law
https://brainly.com/question/1357485
#SPJ4
What is a compound?
a combination of two or more substances that are not chemically combined
a combination of two or more elements that are combined in a certain ratio
Answers
Answer:
a combination of two or more elements that are combined in a certain ratio
Explanation:
A chemical compound is a chemical substance composed of many identical molecules composed of atoms from more than one element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound.
Which of the following is a balanced equation?
Select one:
a. A
b. B
c. C
d. D

Answers
Answer:
B
Explanation:
Balanced equations have the same number of elements on both sides. If the number of elements are equal to each other for every element in the equation on both sides, then the equation is balanced.
Important concept : The big number before an element or compound represents how many molecules of that compound or element there are in a reaction. To find the number of atoms of each element you multiply the coefficient by the subscript ( small number ) which represents the number of atoms of that element in each molecule. Ex. 3H2O. There is a coefficient of 3 meaning that there are 3 molecules of H2O. There is a subscript after H meaning there are 2 atoms of hydrogen in each molecule. To find the total number of atoms we multiply the subscript of hydrogen by the coefficient of the whole molecule. 3 * 2 = 6 , so there are a total of 6 atoms of hydrogen in 3H2O
A) Cu + 2AgNO3 ==> CuNO3 + 2Ag
1 Cu 1
2 Ag 2
2 N 1
3 O 3
The amount of nitrogen atoms is different on both sides of the equation therefore this is not a balanced equation
B) CCl4 + O2 ==> CO2 + 2Cl2
1 C 1
4 Cl 4
2 O 2
The number of atoms of each element is the same on both sides of the equation therefore this is the balanced equation, however lets check the other answer choices just in case.
C) 2K + H2SO4 ==> K2SO4 + 2H2
2 K 2
1 H 4
1 S 1
4 O 4
The number of Hydrogen atoms are different on each side of the equation therefore this is not a balanced equation.
D) 2Al2O3 ==> 2Al + 3O2
4 Al 2
6 O 6
There are a different amount of aluminum atoms on both sides of the equation therefore this is not a balanced equation.
What separates the inner planets from the outer planets in our solar system?
()Comet Belt
()Asteroid Belt
()Their differences
()Distance
Help plss!!
Answers
Answer:
the answer is B Astroid Belt
What does it mean for something to be radioactive?
A. It has an emission spectrum.
B. It is in a stable condition.
C. It generates radio waves.
D. Its nuclei can split apart.
Answers
Answer:
D
Explanation:
Radioactivity means
the emission of ionizing radiation or particles caused by the spontaneous disintegration of atomic nuclei
hope this helps please like and mark as brainliest
(15 POINTS) arrange the conversion factors in order as displayed in the pic

Answers
Answer:
3,1,2
Explanation:
3 bc u go get liters
1 because ur going from C3H8 to CO2
2 because ur getting CO2 liters
Magnesium fluoride can be formed by burning magnesium in fluorine gas. With reference to its bonding, explain why magnesium fluoride has a very high melting point.
Answers
Answer:
Magnesium Flouride is a ionic compound and thus has a giant lattice structure. Its ions are held together in this lattice by strong electrostatic forces of attraction. A large amount of energy is needed to overcome the strong electrostatic forces of attraction between the Mg2+ ions and the F- ions to separate the ions. Hence Magnesium fluoride has a very high melting point.
1.
__________ are the most organized state of matter.
Solids
Liquids
Gases
2.
States of matter change when ________ is added or removed.
Plasma
Energy
3.
Which state of matter has the most movement of its particles?
Solid
Liquid
Gas
Answers
2. Energy. Adding in energy makes the particles of a substance vibrate faster, breaking the forces of attraction between them and removing energy makes the particles vibrate slower and forces of attraction forms between the particles.
3. Gas. The forces of attraction between the particles of a gas are very weak. The particles randomly and quickly move around in all directions.
Solids are the most organized state of matter. States of matter change when energy is added or removed. Gas is the state of matter has the most movement of its particles.
What are the states of matter?The states of matter are the different forms of the matter in which it can exist. They are solids, liquids and gases. Solids are the states of matter in which molecules are very near to each other. They have fixed shape and volume. Liquids is the states of matter in which molecules are a little far from each other. They have different shape and fixed volume. Gas is the states of matter in which molecules are a very far from each other. They have different shape and different volume.
There are two more states of matter which are called as Plasma, Bose Einstein Condensate.
Plasma have highest energy and Bose Einstein Condensate have lowest energy.
Therefore, Solids are the most organized state of matter. States of matter change when energy is added or removed. Gas is the state of matter has the most movement of its particles.
To learn more about States of matter click:
https://brainly.com/question/29069107
#SPJ3
A block of an unknown metal with a mass of 47 g is cooled from 65 °C to 20 °C with a
release of 533 J of heat. From these data, calculate the specific heat of the unknown
metal. (Round to 2 decimal places)
Answers
Answer:313
Explanation:Hope its rught or i calculated wrong
What has a Gibbs free energy of 0?
Answers
Pure elements has Gibbs free energy equal to 0.
The Gibbs free energy is a concept in chemistry specially of thermodynamics. The maximum amount of work that can be accomplished at a constant temperature and pressure by a closed system can be calculated using the Gibbs free energy (also known as Gibbs energy; symbol: denoted as delta G). Additionally, it offers a prerequisite for any processes like chemical reactions that might take place in such circumstances.
When a system achieves equilibrium without being pushed by an input electrolytic voltage, the Gibbs energy is the thermodynamic potential that is reduced. At the equilibrium point, its derivative w.r.t. the system's reaction coordinate vanishes.
To know more about free energy, click here,
brainly.com/question/13765848
#SPJ4
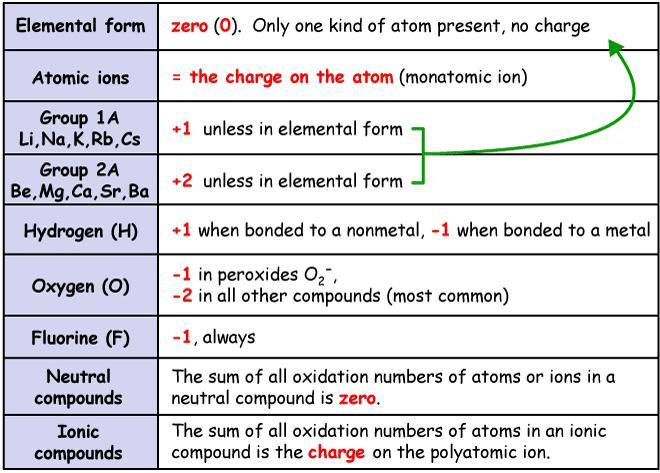
Write the oxidation and reduction half reaction for the equation below, show all work:
Na + Cl = NaCl
Answers
Explanation:
Oxidation in an element occurs when it is losing electrons in the chemical reaction. So it will become more positive in charge.
Reduction occurs when an element gains electrons in the chemical reaction. So it will become more negative in charge.
A good way to remember this is:
OIL RIG= Oxidation Is Losing Reduction Is Gaining
So let's first determine the oxidation number of each element and see how Na & Cl charge in charge to determine if they're being reduced or oxidized.
Na + Cl = NaCl
If we refer to our table, lone elements will always be 0.
0 0
Na + Cl = NaCl
Now we can do the products side where we now have a compound so how did they change now that they're bonded together?
According to our table, group 1 is +1 oxidation number. What about Cl? It has to balance our Na so the overall charge of the compound is neutral (0). So it would be -1.
0 0 +1 -1
Na + Cl = NaCl
Na goes from 0 > +1 where it loses e- > oxidation
Cl goes from 0 > -1 where is gains e- > reduction
So our respective half reactions would be:
Na = NaCl and Cl= NaCl
Its okay if we use the product twice, since it's part of both half reactions.
Science, please help and thank you!!

Answers
The concentration of hydroxide ions is greater than the concentration of hydronium ions for acidic solution. True or false.
Answers
Answer: False
Explanation:
An acid is defined as the substance which looses hydrogen ion or hydronium ions when dissolved in water.
A base is defined as the substance which looses hydroxide ions when dissolved in water.
If the solution has higher hydronium ion concentration as compared to the hydroxide ion concentration, then the pH will be low and the solution will be acidic.
If the solution has low hydronium ion concentration as compared to the hydroxide ion concentration then the pH will be high and the solution will be basic.
Store bought cake mix is usually left to bake at 350°F in an oven, what would happen to the time it
takes for the cake to bake if the temperature was increased by 20°F 2 Explain your answer.
Answers
Answer:
It will reduce
Explanation:
The time it will take to bake the cake will reduce when the temperature is increased by an additional 20°F .
Temperature change has a considerable effect on reaction rates since temperature is directly proportional to the average kinetic energy of reacting particles.
Generally reaction rate varies as temperature directly.
Therefore, the baking time will reduce as temperature increases.
For waste hazardous materials packaged in a lab pack, the inside packaging must be?
Answers
The interior packagings must either be made of glass with a maximum capacity of 4 L (1 gallon) or of metal or plastic with a maximum capacity of 20 L (5.3 gallons).
A chemically suitable absorbent material must be present around inner packaging holding liquid in an amount adequate to absorb the entire liquid content.
physical packing. Only one type of waste material may be contained in each outer package. Except for Division 4.2 Packing Group I materials, which must be packaged in UN standard steel or plastic drums tested and marked to the Packing Group I performance level for liquids or solids, and bromine pentafluoride and bromine trifluoride, which cannot be packaged in UN 4G fiberboard boxes, the following outer packagings are permitted.
a metal drum (UN 1A2, UN 1B2, or UN 1N2), a plywood drum (UN 1D), a fibre drum (UN 1G), a plastic drum (UN 1H2), tested, and designated to at least the Packing Group III performance.
Learn more about Metal here:
https://brainly.com/question/18153051
#SPJ4
Given the following 3D diagram (assume all coordinates are given in cm ) of beam AB, find the reactions at A if the beam is at equilibrium. Assume F
1
is 200 N in the -y direction, F
2
is 300 N, and F
2
follows the line of action created by line BD
Answers
The reactions at A are 152.43 N at point A in the -x direction, 64.04 N at point A in the -z direction, and -382.43 N at point A in the -y direction.
Given a 3D diagram of beam AB, where the forces F1 and F2 are acting on it. F1 has a magnitude of 200 N and acts in the -y direction, whereas F2 has a magnitude of 300 N and follows the line of action created by line BD. The task is to find the reactions at point A if the beam is at equilibrium.The equilibrium of the beam can be understood by the principle of moments and equilibrium. Taking moments of the forces about point A and equating them to zero, we can find the reactions at A. Therefore, we can resolve the forces along the x, y, and z-axis to find the reactions at A.Let the reaction at A in the x-axis be Rax, at y-axis be Ray, and at the z-axis be Raz.
Moments of forces about point A would be:
300 * cos 45° * 5 - 200 * 2 = 5 Rax + 3 Razz component of the force F2 would be:
300 * sin 45° = 212.13 N
Using the equilibrium of forces equation, we get:
Rax = 152.43 N Ray = -382.43 N Raz = 64.04 N
The reactions at A are 152.43 N at point A in the -x direction, 64.04 N at point A in the -z direction, and -382.43 N at point A in the -y direction.
To know more about Moments of forces visit:
brainly.com/question/28977824
#SPJ11
Information gathered from observations and
experimentation is called?
Answers
Answer: Observing
Explanation: Information gathered in an experiment is called data, and its represents observations derived from the methods of the experiment on its sample elements. Hope this helped! :)
The Information gathered from observations and experimentation is called empirical evidence. Empirical evidence is a significant part of scientific research and helps to further results and discussions.
What is scientific research?Scientific research is a well designed and organised steps to conducts a scientific experiment for solving a problem under study. The research methodologies include logical, economic and creative ways to reveal the solution for a problem.
Information obtained through testing or observation is known as empirical evidence. These data are logged and examined by scientists. The procedure, which is a key component of the scientific method, results in the confirmation or rejection of a hypothesis and, as a result, improves our knowledge of the universe.
A hypothesis can be tested and accurately evaluated using different types of data collection, such as experiments that aim to produce a quantifiable or observable reaction, trials that replicate experiments to test their effectiveness or other methods of data collection.
To find more on empirical evidence, refer here:
https://brainly.com/question/21483139
#SPJ2
PLEASE ANSWER ASAP!!! What are examples of Exothermic or Endothermic processes found at home?
Answers
Answer:
Explanation:
Example for Endothermic include melting ice cubes, cooking eggs, or baking breads.
Example for Exothermic include Burning candles, lighting match, or hot packs
natasha wrote a table comparing pure substances and mixtures. what correction should be made to natasha's table? a elements and compounds are considered mixtures. b pure substances can be separated using physical means. c mixtures contain at least three or more substances. d mixtures are substances that are not chemically combined.
Answers
No correction is required in table of pure substances and mixtures.
A mixture contains further than one chemical substance, and thus isn't pure. The substances in a mixture can be rudiments, composites, or both.
Pure substances can be separated using physical means. rudiments and composites are considered fusions. fusions are substances that aren't chemically combined. fusions contain at least three or further substances
A mixture is a physical combination of two or further substances. These aren't combined chemically. The factors retain their individual properties indeed after forming a mixture.
Learn more about fusion visit:
brainly.com/question/29227396
#SPJ4
What type of weathering creates a granite Tor?
Physical or
Chemical or
Biological
Answers
Answer:
Physical
Explanation:
Physical weathering is defined as the geological process in which rocks are broken down but there is no change in their chemical composition.
Granite tor are the rock masses above the ground surface which are associated with granites
Granite Tor is an example of physical weathering as they formed by freeze–thaw weathering. Granite tor are formed when magma present in the crust cools down and form batholith. Rocks above batholith gets erodes and left the batholith to expose.
Hence, the correct answer is "Physical weathering'.
what is the ph of an aqueous solution of 6.99×10-3 m hydrobromic acid?
Answers
The pH of an aqueous solution of 6.99×10⁻³ M hydrobromic acid is approximately 2.15.
The pH of an aqueous solution of 6.99×10-3 M hydrobromic acid is approximately 2.16. This is because hydrobromic acid is a strong acid, meaning that it dissociates completely in water to form H+ ions and Br- ions. The concentration of H+ ions in the solution can be calculated using the formula pH = -log[H+]. Therefore, pH = -log(6.99×10-3) = 2.16.
To determine the pH of an aqueous solution of 6.99×10⁻³ M hydrobromic acid, follow these steps:
1. Identify the formula: Hydrobromic acid (HBr) is a strong acid, meaning it ionizes completely in water, producing H⁺ and Br⁻ ions.
2. Calculate the concentration of H⁺ ions: Since HBr ionizes completely, the concentration of H⁺ ions is equal to the concentration of HBr, which is 6.99×10⁻³ M.
3. Use the pH formula: The pH of a solution is calculated using the formula pH = -log[H⁺], where [H⁺] is the concentration of H⁺ ions in the solution.
4. Calculate the pH: Plug in the concentration of H⁺ ions into the formula:
pH = -log(6.99×10⁻³)
5. Solve for pH: Using a calculator, you will find that the pH is approximately 2.15.
Learn more about pH here:-
https://brainly.com/question/15289741
#SPJ11
1.5g of magnesium ribbon is burnt in oxygen to produce 2.5g of magnesium oxide. How much oxygen is required in grams?
Answers
Answer:
2.5 grams
Explanation:
law of conservation of mass applies. the magnesium completely burns
identify four forms of energy in the following diagram

Answers
Answer:
Chemical, electrical, thermal, & radiant
Explanation:
A battery is a device that stores chenical energy and converts it to electricity. The diagram portrays said electricity powering an incandescent light bulb, which work by using an electrical current to heat a filament until light is produced.
Answer:
1. Light energy
2. Chemical energy
3. Electrical energy
4. Mechancal energy- Potential energy
Explanation:
The electrical energy is converted into light energy to allow the bulb to glow. Batteries are a form of chemical energy ( the flow of electrons), with energy stored in the bonds of molecules contained within the battery acid. The word “stored” — meaning batteries are a form of potential energy ( energy at rest or stored for later use).
classify each element. note that another term for main group is representative, another term for semimetal is metalloid, and the inner transition metals are also called the lanthanide and actinide series. you are currently in a sorting module. turn off browse mode or quick nav, tab to items, space or enter to pick up, tab to move, space or enter to drop. main‑group metal (representative metal) main‑group nonmetal (representative nonmetal) main‑group semimetal (metalloid) transition metal inner transition metal (lanthanide/actinide)
Answers
The classification of the metals is;
Main group/ representative metals - Tl
Main group non metal - Se, Rn
Main group metalloid - As
Transition metal - Mo
Inner transition metal - Ru, Eu
What is the periodic table?The periodic table is a classification of elements according to their atomic numbers. We know that elements are arranged in the periodic table in order of increasing atomic numbers of the elements.
Let us now classify each element as required;
Main group/ representative metals - Tl
Main group non metal - Se, Rn
Main group metalloid - As
Transition metal - Mo
Inner transition metal - Ru, Eu
Learn more about periodic table:https://brainly.com/question/11155928?
#SPJ4

how many grams of hf form from the reaction of 22.2g of nh3 with an excess of fluorine
Answers
When 22.2g of NH₃ reacts with an excess of fluorine, 26.0 g of HF form. The balanced equation for this reaction is: NH₃ + F2 → HF + NHF₂
1. Calculate the molar mass of NH₃ and HF; Molar mass of NH₃ = 14.01 + 1.01 × 3 = 17.04 g/mol Molar mass of HF = 1.01 + 18.99 = 20.00 g/mol
2. Determine the number of moles of NH₃ used. Moles of NH₃ = 22.2 g ÷ 17.04 g/mol = 1.30 mol
3. Find the limiting reactant NH₃ + F₂ → HF + NHF₂
For every mole of NH₃ that reacts with F₂, one mole of HF is produced. Therefore, 1.30 mol of NH₃ will produce 1.30 mol of HF.
4. Calculate the number of moles of HF formed. Number of moles of HF = number of moles of NH₃ used = 1.30 mol5. Calculate the mass of HF formed. Mass of HF = number of moles × molar mass= 1.30 mol × 20.00 g/mol= 26.0 g
Therefore, 22.2g of NH₃ reacts with an excess of fluorine to form 26.0 g of HF.
To know more about fluorine, refer
https://brainly.com/question/15045637
#SPJ11
What is the formula for frequency?
Answers
Answer:
Frequency = Velocity / Wavelength
or
f = v / λ