Answers
Answer:
Explanation:
For answer see attached file .
Related Questions
What is the frequency of UV light that has an energy of 5.2 x 10^-8 J?
Answers
how to calculate molar extinction coefficient with wavelength and absorbance
Answers
The molar extinction coefficient is specific to the substance being measured and the wavelength of light used. Accurate and precise values for absorbance, concentration, and path length are necessary for an accurate calculation.
How to calculate molar extinction coefficient with wavelength and absorbanceTo calculate the molar extinction coefficient (ε) using wavelength (λ) and absorbance (A):
Apply the Beer-Lambert Law: A = εclA is the absorbance, ε is the molar extinction coefficient (in M^-1 cm^-1), c is the concentration of the substance (in M), and l is the path length of the sample (in cm).Rearrange the equation: ε = A / (cl)Ensure that concentration is in molar units (M) and path length is in centimeters (cm).Divide the absorbance by the product of the concentration and path length to obtain the molar extinction coefficient (ε).Learn more on molar extinction coefficient here https://brainly.com/question/31088826
#SPJ1
How many moles of Aluminum are in 54.0 grams of Aluminum (Al)

Answers
Answer:
2 moles!
Explanation:
Hi i hope this helped! I researched it and 2 moles was what came up first.
Which of the following is not a base :CaSO4, Al(OH)3, Ca(OH)2, KOH
Answers
Answer:
among these which is not a base is CaSO4
How much energy is required to lower the temperature of 32.45 grams of water by 4.05 oC?
Answers
According to the specific heat capacity, 37752.33 J of energy is required to lower temperature of water.
What is specific heat capacity?Specific heat capacity is defined as the amount of energy required to raise the temperature of one gram of substance by one degree Celsius. It has units of calories or joules per gram per degree Celsius.
It varies with temperature and is different for each state of matter. Water in the liquid form has the highest specific heat capacity among all common substances .Specific heat capacity of a substance is infinite as it undergoes phase transition ,it is highest for gases and can rise if the gas is allowed to expand.
It is given by the formula ,
Q=mcΔT
It is calculated as, Q=32.45×4.2×277=37752.33 J.
Thus, 37752.33 J of energy is required to lower temperature of water.
Learn more about specific heat capacity,here:
https://brainly.com/question/2530523
#SPJ1
What changes sodium pellets to liquid
Answers
Answer:
when placed in water, a sodium pellet catches on fire as hydrogen gas is liberated and sodium hydroxide forms. chemical change = fire is a sign of chemical reaction.
Explanation:
When placed in water the sodium pellets catch the fire and liberate the hydrogen gas. On mixing with water solid sodium forms a colorless basic solution.
What are the properties of sodium?Sodium is a soft metal. It is a very reactive element with a low melting point. Sodium reacts very quickly with water, snow, and ice to produce sodium hydroxide and hydrogen. It is an alkali metal and the sixth most abundant metal on earth. It has a silvery white color.
It has a strong metallic luster. On reacting with oxygen it produces sodium oxide which on reacting with the water produces sodium hydroxide.
It is used to improve the structure of certain alloys and soaps. It is also used in the purification of metals. Sodium is also present in sodium chloride, an important compound found in the environment.
To learn more about sodium, refer to the link:
https://brainly.com/question/29327783
#SPJ2
5. Water displacement is a method to measure ?
O volume
Odensity
mass
O temperature
PKEASE HELP
Answers
Answer:
density
Explanation:
density, volume holds fluid, and temperature is negligible
Part C
Close the simulation window for carbon dioxide and return to the Cool Molecules Explore page.
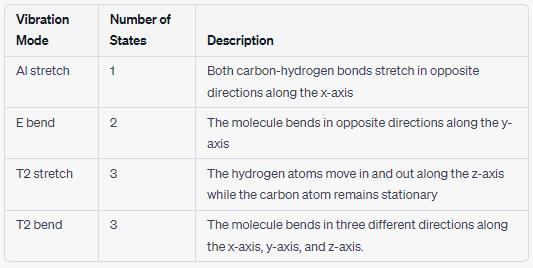
Next, click C and H in the periodic table and repeat the process for methane (CH₂). In the table below, record the names of its vibrational modes
and describe the vibration of the molecule in each mode. Also record the number of unique possible states for each mode.
Vibration Mode
Al stretch
E bend
T2 stretch
T2 bend
Number of States in the:
Mode
Description:
Answers
The vibrational modes and unique possible states for each mode are detailed in the table below.
What is the vibrational mode of a molecule?A vibrational mode of a molecule refers to a specific way in which the atoms inside a molecule can move relative to each other. Molecules are made up of atoms that are connected to each other by chemical bonds, and these bonds act like springs that can vibrate.
When a molecule absorbs energy, it can cause the bonds to stretch, bend, or twist in specific ways, creating different vibrational modes. The vibrational modes of a molecule can provide important information about its structure and chemical properties.
Find out more on vibrational modes here: https://brainly.com/question/30936396
#SPJ1
Please help, its due today! I'll also make you brainiest (put them in an order that's simple, look at the picture and you'll see what I mean) Thank you and God bless! <33
On beaches there are often areas of grassy dunes where people are prohibited from walking. How do these protected areas preserve ecosystem services? Use the graphic organizer to categorize the following as either examples of land reclamation of protecting biodiversity.

Answers
Answer:
Preventing erosion – Land Reclamation
Protecting nesting areas – Protecting Biodiversity
Preventing littering – Land Reclamation
Preventing habitat disruption – Protecting Biodiversity
Protecting native species – Protecting Biodiversity
Preventing contamination of soil – Land Reclamation
Explanation:
I really hope I'm right! I tried my hardest, please give me brainliest :)
have a good day!
Identify the type of reaction and predict the product: Calcium + water -->
Answers
Answer:
Exothermic Reaction
Product = Calcium hydroxide + hydrogen
Explanation:
0.500 mol aluminium hydroxide, Al(OH)3 reacts with 0.500 mol sulphuric acid, H2SO4 to produce aluminium sulphate and water.
a) Write the balanced equation for the reaction.
b) Which reactant is limiting reactant?
c) How many moles of excess reactant is used in the experiment?
d)Determine how many moles of aluminium sulphates was obtained if the percentage yield of aluminium sulphate during the experiment is 77%.
[9 marks]
Answers
a) The balanced equation for the reaction would be as follows:
\(2 Al(OH)_3 + 3 H_2SO_4 ---> Al_2(SO_4)_3 + 6 H_2O\)
b) The mole ratio of aluminum hydroxide to sulfuric acid is 2:3. This means that every 1 mole of the aluminum hydroxide would require 1.5 moles of sulfuric acid.
0.5 mole aluminum hydroxide would require:
0.5 x 3/2 = 0.75 moles of sulfuric acid.
But only 0.500 moles of sulfuric acid is present. Thus, the limiting reagent is sulfuric acid.
c) With 0.5 moles sulfuric acid, the mole of aluminum hydroxide required would be:
0.5 x 2/3 = 0.33
Excess moles of aluminum hydroxide = 0.5 - 0.33
= 0.17 moles
d) The mole ratio of sulfuric acid to aluminum sulfate produced is 3:1. With 0.5 moles sulfuric acid, the mole of aluminum sulfate produced would be:
0.5 x 1/3 = 0.17 moles
But the percentage yield is 77%
77/100 x 0.17 = 0.13 moles
Thus, the moles of aluminum sulfate that would be obtained with a percentage yield of 77% would be 0.13 moles.
More on stoichiometric calculations can be found here: https://brainly.com/question/8062886
The correct answer for the following calculation where 43 and 7 are counted numbers and 2,310 and 0.370 are measured numbers is which of the following? 43 X 2.310 7 X 0.370 a) 38.35 b) 38.4 Oc) 38 O d) 40
Answers
The actual result of the calculation is 101.920. Therefore, none of the options provided in the question matches the correct answer.
To calculate the given expression: (43 × 2.310) + (7 × 0.370), we perform the multiplication first and then add the results.
Multiplying the counted numbers:
43 × 2.310 = 99.330
Multiplying the measured numbers:
7 × 0.370 = 2.590
Now, we add the results:
99.330 + 2.590 = 101.920
The correct answer is not provided in the given options: a) 38.35, b) 38.4, c) 38, or d) 40.
The actual result of the calculation is 101.920. Therefore, none of the options provided in the question matches the correct answer.
It's important to note that when performing calculations, it is crucial to accurately follow the order of operations (multiplication before addition) and ensure precision when dealing with decimal numbers.
In this case, the correct answer is not among the options provided, and the accurate result is 101.920.
For more such questions on calculation visit:
https://brainly.com/question/28902645
#SPJ8
ClO(4)− Express your answer as a chemical formula.
Answers
ClO(4)− is regarded as perchlorate ion and are produced commercially in most situations as salts via industries and in the laboratory.
What is Perchlorate ion?This ion is referred to as a monovalent inorganic anion and is obtained by deprotonation of perchloric acid. It is composed of chlorine and oxygen atoms in the ratio 1 to 4 respectively.
This has 32 valence electrons available in the Lewis structure and is used in the commercial production of solid rocket fuel.This ion has a molar mass of 99.451 g mol−1 and is used in different processes such as an oxidizer and to control static electricity during the process of food preservation in industries.
Therefore ClO₄− is also regarded as perchlorate ion and is the most appropriate choice.
Read more about Perchlorate ion here https://brainly.com/question/16895150
#SPJ1
convert 7.8845 in/sec to ft/min
Answers
1 inch per second (in/s) = 5 foot per minute (ft/min). 7.8845 in/sec is equal to 39.4225 ft/min.
What is unit conversion ?
A unit conversion is the expression of the same property in a different unit of measurement. Time, for example, can be expressed in minutes rather than hours, and distance can be converted from miles to kilometers, feet, or any other length measurement.
We must convert one unit to another in order to achieve accuracy and avoid measurement confusion.
Thus, 1 inch per second (in/s) = 5 foot per minute (ft/min). 7.8845 in/sec is equal to 39.4225 ft/min.
To learn more about the unit conversion, follow the link;
https://brainly.com/question/19420601
#SPJ1
Assuming the reaction is at STP if 2.25 moles of Li are exposed to 3.25 moles of water, which is the limiting reactant?

Answers
Answer: Lithium
Explanation:
The ratio for the reaction of Li and water is the same, but there are more moles of water than lithium. Therefore, lithium is the limiting reactant.
A double replacement reaction is a reaction in which one element replaces a similar element within a compound
True
False
Answers
Answer:
False
Explanation:
. What you’re describing is a displacement reaction.
Answer:
False, that is a single replacement reaction that you have described.
Explanation:
How do you find the protons , neurons and electrons of an element ?
Answers
Answer:
For any element,
Atomic Number (Z) = The number of protons (P⁺) = The number of electrons (e⁻)
Hence,
Z = P⁺ = e⁻
For Neutron,
Mass number (A) – Atomic Number (Z)
N = A – Z
For Examples :-Oxygen atom (O)Atomic Number = 8Proton = 8Electron = 8Mass Number = 16Neutron = 16 – 8 = 8
-TheUnknownScientist 72
True or False: Only objects with energy can perform work.
Answers
Answer: True
Explanation: Physical Quantity which enables an object to do work is Energy. When an object does work, then its work done is only possible when the object has Kinetic Energy, Potential Energy or Both (Mechanical Energy) in it.
pls help i will mark as brainliest

Answers
Answer:
ano po ito?? isa po ba itong story
Balance this equation using oxidation number change method. Show your steps. NH3+O2 -> NO2+H2O
Answers
Answer: The balanced equation is 4 NH3 + 7 O2 --> 4 NO2 + 6 H2O
Explanation:
Oxidation numbers
We begin by noting the oxidation numbers on each atom:
NH3: N = -3, H = +1
O2: O = 0
-----------------
NO2: N = +4, O = -2
H2O: H = +1, O = -2
From this we conclude that N has lost 7 electrons, while O has gained 2.
Half-reactions
Because this case is more complex, we must consider the half-reactions:
Eq. 1) N^-3 --> N^+4 + 7 e- (which says that N has lost 7 electrons)
Eq. 2) O + 2 e- --> O^-2 (which says that O has gained 2 electrons)
However, oxygen is a diatomic atom, so we must adjust the second equation by a factor of 2.
Eq. 1) N^-3 --> N^+4 + 7 e-
Eq. 2) O2 + 4 e- --> 2 O^-2
Balancing the Half-reactions
To balance the half-reactions, we must multiply each equation by a factor that allows us to cancel out the electrons. The LCM of 7 and 4 is 28, so we multiply the first equation by 4, and the second equation by 7, giving:
Eq. 1) 4 N^-3 --> 4 N^+4 + 28 e-
Eq. 2) 7 O2 + 28 e- --> 14 O^-2
Now, adding them together gives:
4 N^-3 + 7 O2 --> 4N^+4 + 14 O^-2
All together...
To complete the reaction, we must add the missing hydrogen atoms. With 4 nitrogen atoms on the left, we require 12 hydrogen atoms to construct the 4 NH3 molecules. We will add 12 H to both sides:
4 N^-3 + 7 O2 --> 4N^+4 + 14 O^-2
+ 12 H + 12 H
-------------------------------------------------------
4 N^-3 + 12 H + 7 O2 --> 4N^+4 + 14 O^-2 + 12 H
We can now combine all the atoms together into the known molecules on each side:
4 NH3 + 7 O2 --> 4N^+4 + 8 O^-2 + 12 H + 6 O^-2
which is,
4 NH3 + 7 O2 --> 4 NO2 + 6 H2O
Please help i have an exam tomorow!!
1. Oxygen is a reactant needed for all _________ reactions.
2. The products of the complete combustion reaction of a hydrocarbon (compound containing carbon and hydrogen) are ______ and _____ .
3. ______ combustion takes place if the quantity of oxygen is sufficient.
4. Incomplete combustion takes place if the quantity of oxygen is _______.
5. Combustion is a ______ change.
6. In a combustion reaction, oxygen is the oxidizer and the substance
which burns is the ______.
7. The lower the kindling temeperature, the _____ is the combustion.
8. If a substance burns at room temperature in the absence of a flame the
combustion is said to be _____.
9. combustion reactions are accompanied by _____ and _____ effect.
10. combustion reactions dont take place at the same _______.
2,6,8, and 10 are the ones i need the most help with
Answers
1. Oxygen is a reactant needed for all combustion reactions.
2. The products of the complete combustion reaction of a hydrocarbon (compound containing carbon and hydrogen) are carbon dioxide and water.
3. Complete combustion takes place if the quantity of oxygen is sufficient.
4. Incomplete combustion takes place if the quantity of oxygen is insufficient.
5. Combustion is a exothermic change.
6. In a combustion reaction, oxygen is the oxidizer and the substance which burns is the fuel.
7. The lower the kindling temperature, the easier is the combustion.
8. If a substance burns at room temperature in the absence of a flame the combustion is said to be spontaneous.
9. Combustion reactions are accompanied by heat and light effect.
10. Combustion reactions don't take place at the same rate.
1)Oxygen is a reactant needed for all combustion reactions. Combustion reactions are chemical reactions that involve the rapid combination of a fuel (usually a hydrocarbon) with oxygen gas. Oxygen acts as the oxidizing agent, providing the necessary component for the reaction to occur. Without oxygen, combustion cannot take place.
2)The products of the complete combustion reaction of a hydrocarbon are carbon dioxide and water. In the presence of sufficient oxygen, hydrocarbons undergo complete combustion, resulting in the production of carbon dioxide (\(CO_2\)) and water (\(H_2O\)). This reaction releases a significant amount of energy in the form of heat and light.
3)Complete combustion takes place if the quantity of oxygen is sufficient. Complete combustion occurs when there is an adequate supply of oxygen available for the reaction. In this case, the fuel (hydrocarbon) reacts completely with oxygen, resulting in the formation of carbon dioxide and water as the only products
4)Incomplete combustion takes place if the quantity of oxygen is limited. In situations where there is insufficient oxygen available, incomplete combustion occurs. This leads to the formation of products such as carbon monoxide (CO) and carbon (soot) in addition to carbon dioxide and water. Incomplete combustion is less efficient and can release harmful pollutants into the environment.
5)Combustion is a chemical change. Combustion is classified as a chemical change because it involves the breaking and forming of chemical bonds between atoms and molecules. The reactants (fuel and oxygen) undergo a chemical reaction to produce new substances (products) with different properties, such as carbon dioxide and water. Heat and light are also typically released during combustion.
6)In a combustion reaction, oxygen is the oxidizer, and the substance that burns is the fuel or combustible material. Oxygen acts as the oxidizing agent, meaning it accepts electrons from the fuel, leading to the oxidation (burning) of the fuel. The fuel provides the carbon and hydrogen atoms that combine with oxygen to form carbon dioxide and water.
7)The lower the kindling temperature, the easier the combustion. The kindling temperature is the minimum temperature at which a substance can ignite and sustain combustion. If the kindling temperature is lower, it means that less heat is required to initiate the combustion process. Substances with lower kindling temperatures are more prone to catching fire and sustaining combustion.
8)If a substance burns at room temperature in the absence of a flame, the combustion is said to be spontaneous. Spontaneous combustion refers to the ignition and burning of a substance without the need for an external ignition source, such as a flame. It occurs when certain materials, under specific conditions, undergo self-heating and eventually reach their ignition temperature, leading to combustion.
9)Combustion reactions are accompanied by heat and light effects. Combustion reactions are highly exothermic, meaning they release a significant amount of heat energy. This energy is released in the form of heat and light, resulting in flames or glowing embers during combustion.
10)Combustion reactions don't take place at the same rate for all substances. The rate of combustion can vary depending on factors such as the nature of the fuel, the availability of oxygen, temperature, and pressure. Different substances have different combustion rates due to variations in their chemical properties and reactivity.
Know more about carbon dioxide here:
https://brainly.com/question/30355437
#SPJ8
What are the molecular weights of the following compounds? 1. MgCl₂ 2. (NH4)SO4
Answers
The molar mass of compound of magnesium chloride is 95.211 g/mole while that of ammonium sulfate is 132.14 g/mole.
What is molar mass?Molar mass of a compound or a molecule is defined as the mass of the elements which are present in it.The molar mass is considered to be a bulk quantity not a molecular quantity. It is often an average of the of the masses at many instances.
The molecular mass and formula mass are used as synonym for the molar mass.It does not depend on the amount of substance which is present in the sample.It has units of gram/mole.
Learn more about molar mass,here:
https://brainly.com/question/12127540
#SPJ1
Initially 100 milligrams of a radioactive substance was present. After 4 hours the mass had decreased by 3%. If the rate of decay is proportional to the amount of the substance present at time t, determine the half-life of the radioactive substance.
Answers
The half-life of the radioactive substance is approximately 2.06 hours.
The half-life of a radioactive substance is the amount of time required for half of the initial amount of the substance to decay. If the rate of decay is proportional to the amount of the substance present at time t, the half-life of the substance can be determined as follows:
Let t be the half-life of the substance, then after t hours, the amount of the substance present will be 100 mg × (1/2) = 50 mg.
At time 4 hours, the amount of the substance present is 100 mg × (1 - 3%) = 97 mg.
Since the rate of decay is proportional to the amount of substance present, the half-life can be calculated as follows: t = 4 hours × (50 mg / 97 mg) = 2.06 hours.
Therefore, the half-life of the radioactive substance is approximately 2.06 hours.
Learn more about half-life:
brainly.com/question/24710827
#SPJ4
Some organisms reproduce asexually and have only one parent. Others reproduce sexually and have two parents. How does the genetic information of a new organism produced through sexual reproduction compare to the genetic information of its parents?
A.It is an exact copy.
B.It is completely different.
C.It is opposite from its parents.
D.It is a blend of the genetic information of its parents.
Answers
Answer:
D.
the father parent shares his genetic information with the mother during sexual intercourse( the sperm and egg)
hydrogen react ls with oxygen at room temperature to form
Answers
Answer:
When molecular hydrogen (H2) and oxygen (O2) are combined and allowed to react together, energy is released and the molecules of hydrogen and oxygen can combine to form either water or hydrogen peroxide.
Consider a car being acted on by balanced forces. Can you conclude whether the car is moving or at rest? Explain your response.
Answers
Since the car is being acted upon by balanced forces, the car is at rest. as the forces are being balanced.
What is a force?
Force is defined as a cause which is capable of changing the motion of an object. It can cause an object which has mass to change it's velocity. It is also simply a push or a pull . It has both magnitude as well as direction.Hence, it is a vector quantity.
It has SI units of Newton and is represented by'F'.Newton's second law states that force which acts on an object is equal to momentum which changes with time. If mass of object is constant, acceleration is directly proportional to net force acting on an object.
The concepts which related to force are thrust and torque .Thrust increases the velocity of an object and torque produces change in rotational speed of an object.
Learn more about force,here:
https://brainly.com/question/28875770
#SPJ6
why is time plotted on the x axis and distance on the y axis?
Answers
Answer:
because time is independent and distance is dependent . time goes on if distance is not there but if distance is covered it is covered in certain time . that's why distance is taken on y axis while displacement on x axis
what part of of the universe is less than 1 km in diameter
Answers
Answer:
Meteoroids
Explanation:
Meteoroids are the elements of the universe that have the smallest diameter among all elements, reaching less than 1 km in diameter. They are any rocky or metallic body, which exists in space and does not reach more than 100 meters. It is common for meteoroids to fall to earth, but their impact is not as destructive as the impact of other space bodies, due to their tiny size.
An infant acetaminophen suspension contains 80.0mg/0.80 mL suspension. The recommended dose is 15 mg/kg body weight. (1.000 lb. is equivalent to 453.59 g; this is a measured equality.)
How many mL of this suspension should be given to an infant weighing 17 lb ? (Assume two significant figures.)
Express your answer using two significant figures.
Answers
The amount, in mL, of the suspension that should be given to an infant weighing 17 lb will be 1.16 mL
Dimensional analysis0.8 mL of the liquid contains 80.0 mg of the drug.
The recommended dose is 15 mg per kg of body weight
The infant to be given the drug weighs 17 lb.
First, let's convert the weight of the infant to kg.
1 lb = 453.59 g
17 lb = 453.59 x 17/1
= 7711.03 g
1000 g = 1 kg
7711.03 g = 7711.03 x 1/1000
= 7.711 kg
So, the baby's weight is 7.711 kg.
The drug dose for the baby can thus be calculated as:
15 mg x 7.711 = 115.67 mg
But 0.8 mL of the drug contains only 80.0 mg. How many mL will contain 115.67 mg?
0.8 x 115.67/ 80.0 = 1.16 mL
More on dimensional analysis can be found here: https://brainly.com/question/13078117
#SPJ1
Which of the following would be classified as a chemical change?
Group of answer choices
A- Breaking glass
B- Water evaporating
C- Burning charcoal
D- Wax melting
Answers
Answer:
C- Burning charcoal
Explanation:
Because burning is a chemical reaction