A_________is the distance from one compression to the next compression in a longitudinal wave. (Page 2 Vocabulary)
A.frequency
B.rarefaction
C.amplification
D.wavelength
Answers
Answer:
wavelength
Explanation:
Related Questions
What can scientists conclude after discovering trilobites?
All early organisms were found in the ocean because they needed oxygen.
Most early organisms were smaller in size.
Most early organisms needed sunlight to survive.
All early organisms were carnivores.
Answers
Answer:
Im pretty sure that it is Most early organisms were smaller in size
Explanation:
Question 4 of 10 For which part of the engineering process would the use of statistics to analyze data about a new drug test be helpful? A. Making a plan B. Identifying a need C. Testing the solution D. Evaluating the results SUBMIT
Answers
The use of statistics to analyze data about a new drug test would be helpful in evaluating the results of the engineering process. Therefore, the answer is D. Evaluating the results.
Statistics can offer insightful information about a new drug's efficacy and safety. Engineers can make choices about whether a medicine is safe and effective for use in people by analysing the data from a drug test to find patterns and trends. This study might also aid in locating any possible hazards or side effects related to the medication.
It is crucial to utilise statistics to assess the outcomes of the engineering process because it enables engineers to make fact-based choices on the efficacy and safety of the medicine. Engineers can also find potential areas for medication improvement or refinement using statistical analysis before the drug is licenced for usage.
Learn more about drug test here
https://brainly.com/question/32030835
#SPJ11
HELP ME ASAP!!!!!!!!!!!!!!!!!!!!!!!!!!

Answers
Answer:
Digestive
Explanation:
if the box weighs 1 500 n how much does the force of gravity do on the box
Answers
The force of gravity acting on the box is 1,500 N or 152.7 kg.
Force of gravity is the force that attracts two objects towards each other. The force of gravity acting on an object is directly proportional to its mass and inversely proportional to the square of the distance between the object and the center of the Earth.
The force of gravity can be calculated using the formula
Fg = mg,
where Fg is the force of gravity, m is the mass of the object, and g is the acceleration due to gravity,
which is approximately 9.81 m/s2 near the surface of the Earth.
If the box weighs 1,500 N, then the force of gravity acting on the box would be
Fg = mg
= (1,500 N)/(9.81 m/s2)
= 152.7 kg.
Therefore, the force of gravity acting on the box is 1,500 N or 152.7 kg.
The force of gravity is an important concept in physics, as it affects everything on Earth. The gravitational force between two objects depends on their masses and the distance between them. The more massive the objects are, the greater the force of gravity between them. Similarly, the closer two objects are, the greater the force of gravity between them.
The force of gravity is also responsible for keeping the planets in orbit around the Sun and the Moon in orbit around the Earth. It is a fundamental force of nature that plays a crucial role in our understanding of the universe.
Learn more about force of gravity from the given link
https://brainly.com/question/2537310
#SPJ11
in which situation is nitrogenase expression up-regulated? choose one: a. low nh4 and low o2 conditions b. high nh4 and high o2 conditions c. low nh4 and high o2 conditions d. high nh4 and low o2 conditions
Answers
Low NH₄ and high O₂ conditions are required for nitrogenase expression up-regulated.
What is nitrogenase expression regulated by?control over the transcription of nitrogenase. The transcriptional control of nitrogen fixing is influenced by the oxygen and ammonium levels in the surrounding air. Because the nitrogenase components are oxygen labile, it is advantageous for bacteria to limit transcription when oxygen concentrations are high.
Reversible mono-ADP-ribosylation of the Fe protein, one of nitrogenase's components, regulates the enzyme's activity. The electrons necessary for the reduction of molecular nitrogen are supplied by this protein to the MoFe protein, the other component.
The enzyme nitrogenase turns nitrogen into ammonia. Some prokaryotes include it. Biological nitrogen fixation is the conversion of dinitrogen to ammonia by living things. The enzyme nitrogenase facilitates the transformation of nitrogen into ammonia.
To know more about nitrogenase expression visit:
https://brainly.com/question/24691243
#SPJ4
how many moles of carbon dioxide are in 211 g of carbon dioxide?
Answers
To determine how many moles of carbon dioxide are in 211 g of carbon dioxide, we'll use the formula of moles given the mass and molar mass of the substance.
What is a mole?
A mole is defined as the amount of a substance that contains as many entities (such as atoms, molecules, or ions) as there are in 12 grams of carbon-12. One mole of a substance contains 6.022 × 1023 entities
To determine the number of moles in a sample, divide the mass of the sample by the molar mass of the substance.
The equation can be written as:
n = m / M,
where n is the number of moles, m is the mass of the sample in grams, and M is the molar mass of the substance in grams per mole.
The molar mass of carbon dioxide is approximately 44 g/mol.
Hence, the number of moles of carbon dioxide in 211 g of carbon dioxide is:
n = m / M
= 211 g / 44 g/mol
= 4.80 moles
Therefore, there are 4.80 moles of carbon dioxide in 211 g of carbon dioxide.
To learn more about moles, visit:
https://brainly.com/question/30885025
#SPJ11
What are interactions between flip flops and sulfuric acid
Answers
Why are intensive properties useful for identifying a substance?
Answers
45.89 g of Hydrogen atoms contains how many moles of Hydrogen
atoms?
Please show work it’s due in 5 minutes I’ll give brainliest
Answers
2.63 g
Explanation:
One way to approach this problem is to determine the percent composition of hydrogen in water.
Describe how matter moves throughout the owl’s food web.
Answers
Your food web should include drawings and labels of the organisms as well as arrows to show how energy (food) moves through the web
What moves throughout the owl’s food web?In the food web in a feed food chain, energy and nutrients go from plants to the fruitarian consuming them, and to the carnivores or omnivores hunt upon the herbivores. owls often eat other carnivores, such as weasels, bats, and astute, and insect-eating birds. Therefore, owls hold a spot at the top of the food chain. Using the food web and simple determining, they track the flow of energy and biomass through the food web, and impose the concept of matter and energy ... food web uses the real-life diet of an owl. Many times in science classes you may vivisect an owl's amount of available energy decrease as it passes through the ecosystem.
So we can conclude that the food web consists of all the food chains in one ecosystem. Each living thing in an ecosystem is part of a different food chain.
Learn more about the food web here: https://brainly.com/question/2179
#SPJ1
Study the image.
Which phrase describes this plate boundary?
does not occur in oceans
may form rift valleys
is a type of convergent boundary
is a region where earthquakes occur
Answers
The phrase that describes the plate boundary shown is D. is a region where earthquakes occur .
What is the plate boundary shown ?A transform boundary is the name given to this tectonic plate boundary. Two or more plates slip past one another in such a border. This shift is typically accompanied by some highly active earthquakes.
At a transform plate border, the grinding motion between the plates causes shallow earthquakes, significant lateral rock displacement, and a wide zone of crustal deformation. Essentially While no new crust is produced, subducted, or formed, and no volcanoes are formed, the fault produces earthquakes.
Find out more on plate boundaries at https://brainly.com/question/22005072
#SPJ1
Nasogastric Intubation and Enteral Feedings: Priority Actions for an Adolescent (RN QSEN - Safety , Active Learning
Template - Nursing Skill, RM FUND RN 9.0 Ch 54)
prepare the formula, tubing, and infusion device.
Check expiration dates, and note the content of the formula.Ensure that the formula is at room temperature.
Set up the feeding system via gravity or pump.
Mix or shake the formula, fill the container, prime the tubing, and clamp it.Assist the client to Fowler's position, or elevate the head of the bed to a minimum of 30°.Auscultate for bowel sounds.monitor tube placement.
Check gastric contents for pH. A good indication of appropriate placement is obtaining gastric contents with a pH between 0 and 4.Aspirate for residual volume.
Flush the tubing with at least 30 mL tap water.Administer the formula
Answers
Nasogastric intubation is a medical procedure in which a flexible tube is inserted through the nose and passed down the throat into the stomach. Enteral feedings refer to the delivery of nutrition directly into the gastrointestinal tract, specifically the stomach or small intestine
To prioritize actions for nasogastric intubation and enteral feedings in an adolescent, the following steps can be taken:
1. Prepare the formula, tubing, and infusion device: Gather all the necessary equipment required for the procedure, including the enteral feeding formula, appropriate tubing, and the infusion device (if using a pump). Ensure that the equipment is clean and in proper working condition.
2. Check expiration dates and note the content of the formula: Verify the expiration dates of the enteral feeding formula and discard any expired products. Take note of the nutritional content and any specific instructions provided by the healthcare provider regarding the formula.
3. Ensure that the formula is at room temperature: Some enteral formulas may require warming to room temperature before administration. Follow the manufacturer's instructions for warming if necessary, ensuring the formula is not too hot to avoid causing discomfort or harm.
4. Set up the feeding system via gravity or pump: Depending on the healthcare provider's order, set up the feeding system using either gravity or a pump. Follow the manufacturer's instructions for assembling the system correctly and securely.
5. Mix or shake the formula, fill the container, prime the tubing, and clamp it: If using powdered formula, mix it with the appropriate amount of water as directed by the manufacturer. Shake the formula well to ensure it is adequately mixed. Fill the container with the prepared formula and connect it to the tubing. Prime the tubing by allowing the formula to flow through it until all the air bubbles are removed. Clamp the tubing to prevent any spillage.
6. Assist the client to Fowler's position or elevate the head of the bed to a minimum of 30°: Position the adolescent in Fowler's position (sitting up at a 90-degree angle) or elevate the head of the bed to at least 30 degrees. This position helps prevent aspiration and facilitates the movement of the formula into the stomach.
7. Auscultate for bowel sounds and monitor tube placement: Use a stethoscope to auscultate the abdomen for bowel sounds. Absence of bowel sounds may indicate potential complications. Additionally, verify the placement of the nasogastric tube by checking for carbon dioxide detection, X-ray confirmation, or following facility-specific protocols.
8. Check gastric contents for pH: Aspirate a small amount of gastric contents using a syringe. Test the pH of the aspirate using pH strips or a pH meter. A pH range between 0 and 4 is considered a good indication of appropriate placement within the stomach.
9. Aspirate for residual volume: To assess gastric residual volume, use a syringe to withdraw the contents of the stomach through the nasogastric tube. Note the amount and appearance of the aspirate, as excessive residual volume may indicate feeding intolerance or delayed gastric emptying.
10. Flush the tubing with at least 30 mL of tap water: After checking gastric residual volume, flush the nasogastric tube with a minimum of 30 mL of tap water to ensure patency and prevent tube occlusion.
11. Administer the formula: Begin the enteral feeding by connecting the tubing to the nasogastric tube and initiating the flow of the formula according to the prescribed rate and schedule. Monitor the client's response during the feeding for any signs of intolerance or complications.
Throughout the procedure, adhere to proper infection control practices, maintain open communication with the adolescent and their family, and provide appropriate education and support.
To know more about incubation and enteral feeding visit:
https://brainly.com/question/15739559
#SPJ11
Fun fact
An apple, potato, and onion all taste the same if you eat them with your nose plugged weird but yeah
Answers
Answer:
oh rlly
Explanation:
didn’t know about that. Hope u have a good day!!
Which number indicates neutral on a pH scale?
A) 1
B) 3
C) 5
D) 7
E) 9
Answers
The number indicates neutral on a pH scale is 7. Therefore the correct option is option E.
The acidity or basicity (alkalinity) of a solution is gauged using the pH scale. With 0 being the most acidic and 14 being the most basic, it has a range of 0 to 14.
Since a solution's pH is 7 or higher, it is regarded as neutral because it is neither acidic nor basic. The pH of pure water at normal temperature is 7, which is regarded as neutral.
While bases have a pH above 7, acids have a pH below 7, with lower numbers suggesting greater acidity and higher numbers indicating greater basicity. Therefore the correct option is option E.
For such more question on neutral:
https://brainly.com/question/24652228
#SPJ11
please help!
Which of the following will an Arrhenius base always produce?
A) H2
B)H2O
C)H+
D)OH-
Answers
Answer: D. OH- (Edmentum)
Explanation: Took the test and got it right!
Which designation does NOT correlate molecule's mass with its correct associated units?
-Mr; Da
-m; kDa
-u; g
-m; MDa
Answers
The designation "Mr" does not correlate a molecule's mass with its correct associated units, as it is a relative measure of molecular mass and not expressed in a specific unit like Da, kDa, or g. Thus, Option A is correct.
"Mr" stands for relative molecular mass and is a dimensionless value that represents the mass of a molecule relative to the mass of a carbon-12 atom. In contrast, "Da" (Dalton) and "kDa" (kilodalton) are units of mass commonly used to express the molecular weight of a molecule, while "g" (gram) is a standard unit of mass. "MDa" (megadalton) is also a unit of mass, but it is a larger unit than "kDa."
Therefore, only "Mr; Da" does not provide a specific unit for the mass of a molecule and cannot be used to directly convert to other units.
Learn more about molecule's mass brainly.com/question/24191825
#SPJ11
What speed covers 20 miles in 25 mins?
Answers
Answer:
48 mph
Explanation:
divide time by distance - 20/25 = .8 miles per minute, multiple by 60 to get mph
Suppose in the lcy Hot lab that the burner transfers 325 kJ of energy to 450 g of liquid water at 20. 'C. What mass of the water would be boiled away? Suppose in the lcy Hot lab that the burner transfers 325 kJ of energy to 450 g of liquid water at 20. 'C. What mass of the water would be boiled away?
Answers
Each liter of air has a mass of 1.80 grams. How many liters of air are contained in
2.5 x 10^3 kg of air?
Answers
HELPPPPP PLEASEEE
Given that 2H20 + 2H2 + O2 is the balanced equation.
Use the balanced equation to solve the following:
a. Moles of oxygen gas produced if 200 mL of water decomposed completely. Note that the density of water is 1 g/mL.
b. Moles of hydrogen gas produced if 200 mL of water decomposed completely. Note that the density of water is 1 g/mL.
c. Molecules of oxygen gas produced if 200 mL of water decomposed completely. Note that the density of water is 1 g/mL.
Answers
1) 0.0089 moles of hydrogen is produced
2) 0.00445 moles of oxygen is produced
3) 2.7 * 10^21 molecules of oxygen is produced
What is the balanced reaction equation?We can see form the question that the reaction equation is;
2H20 ----> 2H2 + O2
The number of moles of water can be obtained from;
1 mole of water would have a volume of 22400 mL
x moles of water would have a volume of 200 mL
x = 200 * 1/22400
x = 0.0089 moles
If 2 moles of H2 produced 2 moles of H2 from the equation then the moles of H2 produced is 0.0089 moles.
If 2 mole of water produced 1 mole of oxygen
0.0089 moles of water produced x moles of oxygen
x = 0.00445 moles
The number of the molecules of oxygen produced is obtained from;
1 mole of oxygen contains 6.02 * 10^23 molecules
0.00445 moles contains 0.00445 moles * 6.02 * 10^23 molecules/1 mole
= 2.7 * 10^21 molecules
Learn more about moles:https://brainly.com/question/31597231
#SPJ1
What is Decomposition Reaction
Answers
Answer:
Explanation:
Decomposition reaction, also known as analysis or dissociation, is a type of chemical reaction in which a compound breaks down into simpler substances or elements. In this reaction, a single reactant undergoes a chemical change and produces two or more products.
The decomposition reaction can be represented by the general equation:
AB → A + B
Where AB is the reactant, and A and B are the products. The reactant AB is usually a compound, and it breaks down into its constituent elements or simpler compounds.
There are different types of decomposition reactions, including:
Thermal decomposition: It occurs when a compound is heated, resulting in its decomposition into simpler substances. For example, the thermal decomposition of calcium carbonate (CaCO3) produces calcium oxide (CaO) and carbon dioxide (CO2):
CaCO3 → CaO + CO2
Electrolytic decomposition: It takes place when an electric current is passed through an electrolyte, causing it to break down into its component ions. For instance, the electrolysis of water (H2O) leads to the decomposition into hydrogen gas (H2) and oxygen gas (O2):
2H2O → 2H2 + O2
Photochemical decomposition: It occurs when a compound undergoes decomposition due to exposure to light energy. Chlorine gas (Cl2) can decompose into chlorine atoms (Cl) under the influence of light:
Cl2 → 2Cl
These are just a few examples of decomposition reactions. They are important in various chemical processes and are used in industries, laboratory experiments, and natural phenomena. By understanding and controlling decomposition reactions, scientists can gain insights into the behavior of different compounds and develop practical applications in fields such as chemistry, materials science, and environmental science.
Answer:
Explanation:
reaction in which a compound breaks down into simpler substances or elements
Which is one way that scientists ensure that data is reliable? what is the answer
Answers
Explanation:
by doing the experiment
to test the hypothesis of the problems.
What period is the atom in?
What group is the atom in?
What is the name of this atom?
What other atom would have similar properties
to the atom?
Would this atom be malleable or brittle?

Answers
It is in group 17
It is a chlorine atom because it has 17 electrons which means the atomic number is 17
questionyou heat two substances, a and b. both substances change color. when cooled, both substances return to their original colors.what most likely happened in this situation?
Answers
Two substances, a and b were heated. Both substances change color. when cooled, both substances return to their original colors.
What is a physical change and examples?Changes within the size or form of matter are examples of physical change. Physical changes include transitions from one state to a different , like from solid to liquid or liquid to gas. Cutting, bending, dissolving, freezing, boiling, and melting are a number of the processes that create physical changes.
Why it's a physical change?Physical changes occur when objects or substances undergo a change that doesn't change their chemical composition. This contrasts with the concept of chemical process in which the composition of a substance changes or one or more substances combine or break up to form new substances.
Learn more about physical change:
brainly.com/question/960225
#SPJ4
How many grams of sodium hydroxide (NaOH) do you need to make 6.5 L of 1.0M solution?
Answers
260grams
Explanations:The formula for calculating the moles of NaOH is expressed as
\(\begin{gathered} moles=molarity\times volume \\ moles\text{ of NaOH}=1.0M\times6.5L \\ moles\text{ of NaOH}=6.5moles \end{gathered}\)Determine the required mass of NaOH
\(\begin{gathered} Mass\text{ of NaOH}=moles\times molar\text{ mass} \\ Mass\text{ of NaOH}=6.5\cancel{moles}\times\frac{40g}{\cancel{mole}} \\ Mass\text{ of NaOH}=260grams \end{gathered}\)Hence the grams of sodium hydroxide (NaOH) you need to make 6.5 L of 1.0M solution is 260grams
Who wants to help me ???
Answers
Answer:
what do u need help tho
Explanation:
What will the original pressure be for an unknown sample of gas at 23 mL and 41 Kelvin if the new measurements indicate the following: 42 mL at 13 atm and 154 kelvin?
Answers
The original pressure of the unknown gas sample is approximately 6.26 atm when the initial volume is 23 mL and the initial temperature is 41 Kelvin, based on the measurements indicating a final volume of 42 mL at 13 atm and 154 Kelvin.
To determine the original pressure of the unknown gas sample, we can use the combined gas law equation:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
Where:
P1 = Original pressure (to be determined)
V1 = Original volume (23 mL)
T1 = Original temperature (41 Kelvin)
P2 = Final pressure (13 atm)
V2 = Final volume (42 mL)
T2 = Final temperature (154 Kelvin)
Substituting the given values into the equation:
(P1 * 23 mL) / (41 K) = (13 atm * 42 mL) / (154 K)
Simplifying the equation:
(P1 * 23 mL * 154 K) = (13 atm * 42 mL * 41 K)
Now, solving for P1 (the original pressure):
P1 = (13 atm * 42 mL * 41 K) / (23 mL * 154 K)
Calculating the expression:
P1 ≈ 6.26 atm
Therefore, the original pressure of the unknown gas sample is approximately 6.26 atm when the initial volume is 23 mL and the initial temperature is 41 Kelvin, based on the measurements indicating a final volume of 42 mL at 13 atm and 154 Kelvin.
for more questions on pressure
https://brainly.com/question/24719118
#SPJ11
at constant temperature, a sample of helium at 760. torr in a closed container was compressed from 5.00 l to 3.00 l. what was the new pressure exerted by the helium on its container? at constant temperature, a sample of helium at 760. torr in a closed container was compressed from 5.00 l to 3.00 l. what was the new pressure exerted by the helium on its container? 1 270 torr 800. torr 3 800 torr 2 280 torr 15.0 torr
Answers
At this point, helium is exerting 1270 torr of pressure against its container.
What is Boyle's Law?Boyle's Law, a cornerstone of chemistry, describes the behavior of a gas sustained at a constant temperature. Robert A. Boyle discovered this law in 1662: at a certain temperature, the volume of a gas is inversely proportional to the pressure it exerts. To put it another way, if a gas is driven into a closed place, it will condense to fit the area but raise the pressure on the container.
Boyle's law, which is arguably a clearer way to put it, is the relationship between pressure and volume. Mathematically, Boyle's law can be written as pV=k, where p stands for the pressure, volume, and mass of the gas and k is a constant.
The solution is straightforward when temperature and moles are constant,
P₁V₁ = P₂V₂
Given,
P₁ = 760 torr
P₂ = ?
V₁ = 5.00 I
V₂ = 3.00 I
(760)(5.00) = (3.00)(P₂),
P2 = 1270 torr.
To know more about Boyle's Law, visit:
https://brainly.com/question/1437490
#SPJ4
How much calcium oxide would be made by the thermal decomposition of 25 grams of calcium carbonate?
CaCO3 -> CaO + CO2

A. 28 grams
B. 12 grams
C. 14 grams
D. 25 grams
Answers
Answer:
14 grams of calcium oxide would be produced by thermal decomposition of 25 grams of calcium carbonate.
Explanation:
You know:
CaCO₃ → CaO + CO₂
In the first place, by stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction) the following quantities react and are produced:
CaCO₃: 1 moleCaO: 1 moleCO₂: 1 moleBeing:
Ca: 40 g/moleC: 12 g/moleO: 16 g/molethe molar mass of the compounds participating in the reaction is:
CaCO₃: 40 g/mole + 12 g/mole + 3*16 g/mole= 100 g/moleCaO: 40 g/mole + 16 g/mole= 56 g/moleCO₂: 12 g/mole + 2*16 g/mole= 44 g/moleThen, by stoichiometry of the reaction, the following mass amounts of the compounds participating in the reaction react and are produced:
CaCO₃: 1 mole* 100 g/mole= 100 gCaO: 1 mole* 56 g/mole= 56 gCO₂: 1 mole* 44 g/mole= 44 gYou can then apply the following rule of three: if by stoichiometry of the reaction 100 grams of calcium carbonate CaCO₃ produce 56 grams of calcium oxide CaO, 25 grams of CaCO₃ how much mass of CaO will it produce?
\(mass of calcium oxide=\frac{25 grams of CaCO_{3} *56 grams of CaO}{100 grams of CaCO_{3} }\)
mass of calcium oxide= 14 grams
14 grams of calcium oxide would be produced by thermal decomposition of 25 grams of calcium carbonate.
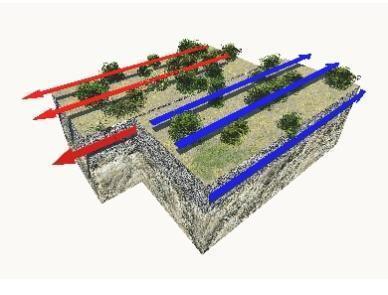
Rainfall over land often results in water runoff and infiltration. Explain how runoff differs from infiltration.
Plz help T^T
Answers
Answer:
Runoff is water from precipitation that along with the ground level to water bodies while infiltration is water that seeps through into the soil and could possibly become groundwater.
Explanation:
Normally, where there are more cities and capitals and fewer trees, there would be more runoff and flooding would happen more frequently. In the countryside, there would be more trees and roots that would absorb the water from the soil and thus there would be more infiltration.