At what temperature does water freeze?
Answers
Answer:
32 degrees
Explanation:
Related Questions
How many miles are contained in 48.41L of Ne
Answers
We can see from the calculation of the number of moles of the neon that we are going to have about 2.2 moles
What is the mole?The mole is a unit of measurement for substance amounts in chemistry. One mole is the volume of a substance that contains the same number of elementary particles as there are in 12 grams of carbon-12. These particles can be atoms, molecules, ions, or electrons. Avogadro's number, or about 6.022 x 1023 particles per mole, is the quantity of things.
We know that;
1 mole of the Ne occupies 22.4 L
x moles will occupy 48.41L
x = 2.2 moles
Learn more about moles:https://brainly.com/question/26416088
#SPJ1
What are some applications of fission reactions? as a zero-waste energy source for generating large amounts of heat for creating stable elements from unstable ones for creating new, heavier elements as the energy source in nuclear weapons
Answers
Some applications of fission reactions are as a zero-waste energy source for generating large amounts of heat and as the energy source in nuclear weapons.
What are nuclear fission reactions?Nuclear fission reactions are reactions in which the nucleus of heavier atoms are split into two or more atoms of smaller nucleus with the release of energy and radiation.
Some of the applications of fission reactions are as a zero-waste energy source for generating large amounts of heat and as the energy source in nuclear weapons.
Learn more about fission reactions at: https://brainly.com/question/3992688
#SPJ4
the stanley miller apparatus demonstrated that organic molecules could assemble spontaneously in an environment lacking free oxygen and containing water, methane, and ammonia in the presence of an abundant energy source, such as an electric discharge. the research was considered supportive of the organic soup hypothesis, which states that the primitive atmosphere provided inorganic precursors from which organic molecules could have been synthesized in the presence of an energy source. based on subsequent research, the primordial atmosphere was determined to contain less methane and more carbon dioxide. the new data about the composition of the early atmosphere had which of the following effects on origin-of-life hypotheses?
Answers
The Stanley miller apparatus demonstrated , The new data about the composition of the early atmosphere had which of the following effects on origin-of-life hypotheses : A new organic soup hypothesis was proposed to account for the new data for the atmosphere.
The Stanley miller hypothesis proposed the evolution of life on the earth. from the several theories the acceptance is on soup theory. The organic soup theory proposed the new data for the atmosphere also called as primordial soup theory.
Thus, The Stanley miller apparatus demonstrated , The new data about the composition of the early atmosphere had which of the following effects on origin-of-life hypotheses : A new organic soup hypothesis was proposed to account for the new data for the atmosphere.
To learn more about the Stanley miller here
https://brainly.com/question/28235998
#SPJ4
N each reaction box, place the best reagent or reactant from the list supplied. Stoichiometry is omitted
Answers
Stoichiometry is omitted reactant. Reactants are the substances that participate in a chemical reaction. The process by which atoms, the fundamental building blocks of matter.
Rearrange themselves to form new combinations is described by a chemical reaction. Reactants - Reactants are the substances that participate in a chemical reaction. Products - Substances formed as a result of the formation of new bonds in a chemical reaction are referred to as products. As an example: H2 and O2 are reactants in this case because they participate in the chemical reaction. Reactants are the starting materials and appear on the left side of the equation. The products of the reaction are written on the right-hand side of the equation.
To learn more about Reactants, click here.
https://brainly.com/question/17096236
#SPJ4
According to the N+1 rule, a hydrogen atom that appears as a quartet would have how many neighbor H's?3458Arrange the following light sources, used for spectroscopy, in order of increasing energy (lowest energy to highest energy)- 1. 2. 3. 4.infrared- 1. 2. 3. 4.ultraviolet- 1. 2. 3. 4.visible- 1. 2. 3. 4.radio wavesUsing the spin-spin coupling, one can determine the number of neighbor H's a particular hydrogen atom has.TrueFalse
Answers
The statement "Using the spin-spin coupling, one can determine the number of neighbor H's a particular hydrogen atom has" is true.
According to the N+1 rule, a hydrogen atom that appears as a quartet would have three neighboring hydrogen atoms. This rule states that the number of peaks in a hydrogen NMR signal is equal to the number of neighboring hydrogen atoms plus one.
The correct order of increasing energy for the listed light sources used for spectroscopy is as follows:
Radio waves (lowest energy)
Infrared
Visible
Ultraviolet (highest energy)
Spin-spin coupling is a phenomenon observed in nuclear magnetic resonance (NMR) spectroscopy.
It occurs when the magnetic field generated by one nucleus affects the magnetic field experienced by another nearby nucleus, resulting in the splitting of NMR signals into multiple peaks.
By analyzing the splitting pattern, one can determine the number of neighboring hydrogen atoms a particular hydrogen atom has.
Therefore, the statement "Using the spin-spin coupling, one can determine the number of neighbor H's a particular hydrogen atom has" is true.
To know more about spectroscopy, refer here:
https://brainly.com/question/28543039#
#SPJ11
the binding of small effector molecules, protein-protein interactions, and covalent modifications are three common ways to modulate the activities of transcription factors. which of these three mechanisms are used by steroid receptors and by the creb protein?
Answers
Protein-protein interactions and effector molecule binding at steroid receptors; covalent modification and protein-protein interactions at CREB protein.
How a molecule is formed?A molecule is the term used to describe the aggregate of atoms that results from the formation of covalent bonds. Therefore, we might claim that a monomer is the most basic component of a covalent composite. In nature, oxygen exists as a molecule. Dioxygen is created when two oxygen atoms make a covalent triple bond with one another.
Is water a molecule?Molecules are created when atoms come together. 2 hydrogen (H) atoms one and oxygen (O) atom make up the three atoms that make up a water molecule. Because of this, water is occasionally abbreviated as H2O. There are billions of hydrogen atoms in an one drop of liquid.
To know more about Molecule visit:
https://brainly.com/question/28238455
#SPJ4
which of the following is a pure substance
1 heterogenous mixture
2 homogenous mixture
3 solution
4 element
Answers
Answer:
2 homogenous mixture
Explanation:
Which statement best describes a physical change?

Answers
Answer:
the answer is C.Changes can occur to certain physical properties of the substance,
Explanation:
a physical change occurs when the physique of a substances is changes while its composition remain unchanged. for example when the ice melts it is a physical change as the shape of the ice is changing but the properties of both ice and water are same.
can there be an ionic bonding between 2 metals or 2 non-metals?
Answers
Answer:
No
Explanation:
Ionic bonding is where an one atom wants to gain e- while the other wants to lose e-
Such as NaCl.
2. How many moles are in 7.30 X 10^23 molecules of NaCl?
Answers
Answer:
\( \huge{ \boxed{1.213 \: \text{moles}}}\)
Explanation:
To find the number of moles in a substance given it's number of entities we use the formula
\( \bold{n = \frac{N}{L}} \\ \)
where
n is the number of molesN is the number of entitiesL is the Avogadro's constant which is 6.02 × 10²³ entitiesFrom the question
N = 7.30 × 10²³ NaCl molecules
\(n = \frac{7.30 \times {10}^{23} }{6.02 \times {10}^{23} } = \frac{7.30}{6.02} \\ = 1.2126\)
We have the final answer as
1.213 molesOrganelle X is a -
A.
mitochondrion, which makes energy for the cell.
B.
ribosome, with carries proteins within the cell.
C.
centriole, which helps divide the cell in two.
D
lysosome, which helps digest substances inside the cell.

Answers
Answer:
A.
Explanation:
PLEASE HELP
Which shows an isomer of the molecule below?
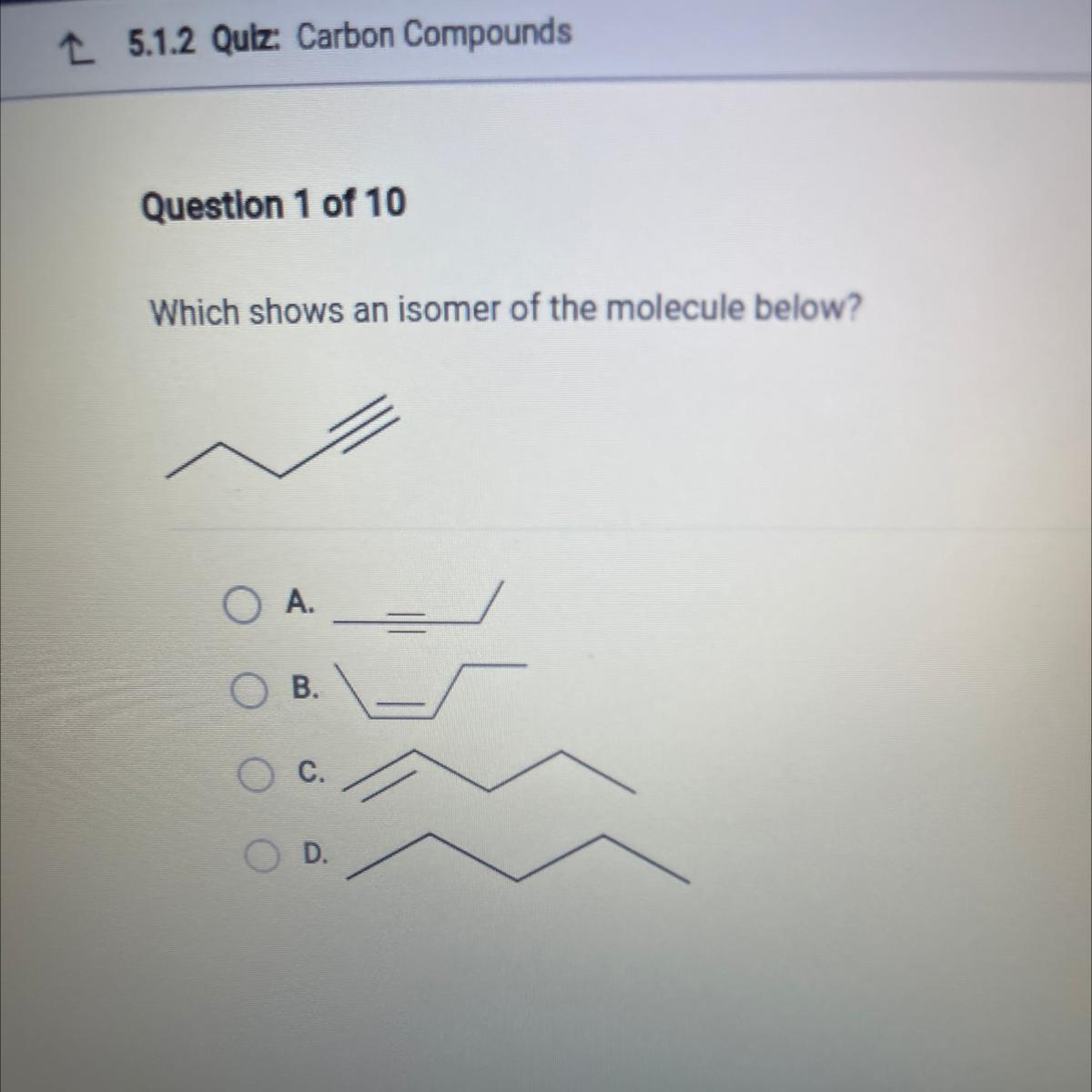
Answers
2. The change in internal energy for the expansion of a gas sample is -4750 J. How much work is done if the gas sample loses 1125 J of heat to the surroundings? Is this work done by the gas or done by the surroundings?
Answers
Answer:
The work done by the gas expansion is 5875 J,
Since the work done is positive, the work is done by the gas on the surroundings.
Explanation:
Given;
change in internal energy, ΔU = -4750 J
heat transferred to the system, Q = 1125 J
The change in internal energy is given by;
ΔU = Q - W
Where;
W is the work done by the system
The work done by the system is calculated as;
W = Q - ΔU
W = 1125 - (-4750)
W = 1125 + 4750
W = 5875 J
Since the work done is positive, the work is done by the gas on the surroundings (energy flows from the gas to the surroundings).
Therefore, the work done by the gas expansion is 5875 J
determine a measurement that might be more useful thanmeasuring the movement of an individualcharge
Answers
A coulomb is a measurement that might be more useful in measuring the movement of an individual charge.
What do you mean by coulomb ?A coulomb is defined as the amount of charge that passes through an electrical conductor transporting one ampere per second.
The SI unit of electric charge is the coulomb. It is derived to SI unit and is corresponded to the symbol C.
Electric current is the flow of electric charge through with an object. The most common charge bearers are the positively charged proton and the negatively charged electron.
Thus, A coulomb is a measurement that might be more useful in measuring the movement of an individual charge.
To learn more about the coulomb, follow the link;
https://brainly.com/question/12498766
#SPJ1
a 50.00-ml solution of 0.100 m hcl is titrated with 0.150 m naoh. what is the ph at the equivalence point?
Answers
The pH at the equivalence point will be 7. To find the pH at the equivalence point of the titration, we need to determine the moles of HCl and NaOH and then calculate the concentration of the resulting salt solution.
First, let's calculate the moles of HCl:
Moles of HCl = volume (in L) × molarity
= 0.050 L × 0.100 mol/L
= 0.005 mol
Since the stoichiometric ratio between HCl and NaOH is 1:1, the moles of NaOH used will also be 0.005 mol.
At the equivalence point, all the HCl is neutralized by the NaOH. Therefore, we have a solution of NaCl.
The volume of the resulting solution will be the sum of the initial volumes of HCl and NaOH:
Volume of resulting solution = Volume of HCl + Volume of NaOH
= 0.050 L + 0.050 L
= 0.100 L
To find the concentration of the resulting NaCl solution, we divide the moles of NaCl by the volume:
Concentration of NaCl = moles of NaCl / volume of NaCl solution
= 0.005 mol / 0.100 L
= 0.050 mol/L
NaCl is a salt of a strong acid (HCl) and a strong base (NaOH). It completely dissociates in water, resulting in a neutral solution. Therefore, the pH at the equivalence point will be 7.
To know more about pH visit-
brainly.com/question/28580519
#SPJ11
How many grams of KClO3 will dissolve in 300g of water at 50°C?
Answers
Answer:
50 g
Explanation:
Please brainiest
state and appearance sodium chloride
Answers
Sodium chloride is white, crystalline solid at room temperature.
Sodium chlorideIt is a compound whose components atoms are sodium and chlorine.
The atoms of the two elements combine chemically through an ionic bond. The sodium donates it valence electron to the chlorine atom.
Thus, sodium chloride is an ionic compound. It has a characteristic white appearance and crystalline structure.
The compound is a solid at room temperature. It has a melting point of 801 °C.
More on sodium chloride can be found here: https://brainly.com/question/9811771
#SPJ1
Which of the following is an example of class evidence?
Group of answer choices
DNA
Fingerprints
Retina scan
Blood type
Answers
A piece of evidence obtained from crime scenes that might have either class or individual characteristics is called a class evidence. One of the example for class evidence is blood type. Thus, option d is correct.
What is class evidence ?Class evidences are characteristics of physical evidence that can only ever be connected to a collection of sources rather than a single one. Evidence that is found to have class characteristics may be used to narrow the pool of possibilities, but it cannot be tied specifically to one person or source.
Class evidences can show that only a small group of persons possessed all the traits linked to criminal activity proof. Individual evidence, on the other hand, consists of items that are connected to a specific individual.
Examples of class evidence include fires, paint, and blood type. Therefore, option d is correct.
Find more on class evidences:
https://brainly.com/question/14263276
#SPJ6
hello people ~
Metals like zinc and aluminium react with sodium hydroxide to produce ____ gas.
A. hydrogen
B. hydrogen sulphide
C. oxygen
D. sulphur dioxide
Answers
Answer:
it's hydrogen
Explanation:
ig it's correct
What is an isotope of the same element?
Answers
Answer:
please mark as brainliest
Explanation:
Isotopes are atoms of the same element that have different numbers of neutrons but the same number of protons and electrons . The difference in the number of neutrons between the various isotopes of an element means that the various isotopes have different masses.
example:chlorine is an example of an isotope it has a proton number of 17 and a mass number of 35 in some cases they have a proton number of 17 and a mass number of 37 there is difference in the number of neutron to calculate this we do it this way.for the first one
mass number=proton+neutron
neutron=mass number-proton
neutron=35-17
neutron=18
for the second one
neutron=37-17
neutron=20
When water reacts with potassium metal the hydrogen produced ignites explosively on the surface of water .What cause the ignition
Answers
Answer:
Pottasium reacts with water vigorously and the reation is exothermic. The heat released causes the hydrogen released to ignite
Explanation:
characteristic of a chemical compound
Answers
Answer:
Hello Adam Here!!!
Explanation:
A chemical compound has the following characteristics: (i) A chemical compound is obtained by the chemical combination of two or more elements in a definite proportion by mass. (ii) Compounds are homogeneous, i.e. their properties are the same throughout.
Happy to Help! =)
Someone plz help me !!
Science
Or
Bias?

Answers
For an atom of sulfur, there are?
1. two electron shells with 6 valence electrons
2. three electron shells with 6 valence electrons
3. four electron shells with 6 valence electrons
4. five electron shells with 6 valence electrons
Answers
Answer:
I think its b, but ik not completely sure.
Answer:
I think the second one...
1. Starting with a 0. 1525 m hcl stock solution, three standard solutions are prepared by sequentially diluting 5. 00 ml of each solution to 100. 0 ml. What is the concentration of each solution?.
Answers
Each solution has a concentration of 7.625 x 103 m, 3.8125 x 104 m, and 1.90625 x 105 m, respectively.
Dilution is the process of lowering a sample's concentration by incorporating more solvent, according to its definition. The following is the dilution formula.
C₁V₁ = C₂V₂
where C1 is the sample's initial concentration.
V1 is the initial sample volume.
After dilution, the ultimate concentration is C2.
After dilution, the ultimate total volume is V2.
Calculate the concentration of the diluted solution by entering the numbers into the formula.
first common answer
C₁V₁ = C₂V₂
0. 1525 m(5.00 ml) = C₂(100.0 ml) (100.0 ml)
C₂ = 7.625 x 10⁻³ m
Second common solution
C₁V₁ = C₂V₂
7.625 x 10⁻³ m(5.00 ml) = C₂ (100.0 ml)
C₂ = 3.8125 x 10⁻⁴ m
Third accepted option
C₁V₁ = C₂V₂
3.8125 x 10⁻⁴ m(5.00 ml) = C₂(100.0 ml) (100.0 ml)
C₂ = 1.90625 x 10⁻⁵ m
To learn more about dilution Visit:
brainly.com/question/1615979
#SPJ4
Question 2 please I mark brainliest

Answers
Answer: C is the answer
Calculate the mass of solid NaCl that must be added to 1.50 L of a 0.100 M AgNO3 solution to precipitate all the Ag+ ions in the form of AgCl.
Answers
Answer:
0.1169g .
Explanation:
1. Multiply the concentration (0.5 mols/Liters) by the volume of solution you want (0.5 Liters) to find the moles of NaCl you need. 2. Multiply the moles of NaCl by its molar mass (58.44 g/mol) to find the grams of solute needed.
A sample of hexane (C6H14) has a mass of 0.580 g. The sample is burned in a bomb calorimeter that has a mass of 1.900 kg and a specific heat of 3.21 J/giK. What amount of heat is produced during the combustion of hexane if the temperature of the calorimeter increases by 4.542 K? Use q equals m C subscript p Delta T..
Answers
The mass of the hexane sample is 0.580 g. The mass of the bomb calorimeter is 1.900 kg, and its specific heat is 3.21 J/g·K. The change in temperature is 4.542 K. So, heat produced during the combustion of hexane is 11195.1576 J.
To calculate the amount of heat produced during the combustion of hexane, we can use the formula q = m × C × ΔT.
First, we need to determine the mass of the hexane sample, which is given as 0.580 g.
Next, we need to find the mass of the bomb calorimeter, which is given as 1.900 kg.
Note that we need to convert the mass to grams by multiplying it by 1000, so the mass is 1900 g.
The specific heat of the bomb calorimeter is given as 3.21 J/g·K.
Finally, we have the change in temperature, which is given as 4.542 K.
Now, we can substitute these values into the formula: q = (0.580 g + 1900 g) × 3.21 J/g·K × 4.542 K.
Simplifying the equation, we get:
q = 2467.8 J/g·K × 4.542 K
Calculating the product:
q = 11195.1576 J
To know more about combustion refer to this:
https://brainly.com/question/31123826
#SPJ11
Al (s) + O2 (g)→ Al2O3 (s) what type of chemical reaction is it
Answers
Explanation:
Correct option is
D
3
The unbalanced chemical reaction is Al(s)+O
2
(g)→Al
2
O
3
(s)
Balance Al atoms by adding 2 to Al on LHS
2Al(s)+O
2
(g)→Al
2
O
3
(s)
Balance O atoms by adding
2
3
to O
2
on LHS
2Al(s)+
2
3
O
2
(g)→Al
2
O
3
(s)
Multiply the whole equation with 2.
4Al(s)+3O
2
(g)→2Al
2
O
3
(s)
This is the balanced chemical equation. The coefficient of O
2
is 3.
Freon, a very useful refrigerant, is produced in the following reaction:
3CCl4 (g) + 2SbF3 (s) → 3CCl2F2 (g) + 2 SbCl3 (s)
If a chemist wants to make 3.0 x 106 moles of Freon using excess carbon tetrachloride, how many moles of antimony trifluoride will the chemist need?
Answers
Answer:
The chemistry will need 2*10⁶ moles of antimony trifluoride.
Explanation:
The balanced reaction is:
3 CCl₄ (g) + 2 SbF₃ (s) → 3 CCl₂F₂(g) + 2 SbCl₃ (s)
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of each compound participate in the reaction:
CCl₄: 3 moles SbF₃: 2 moles CCl₂F₂: 3 molesSbCl₃: 2 molesYou can apply the following rule of three: if by reaction stoichiometry 3 moles of freon are produced by 2 moles of antimony trifluoride, 3*10⁶ moles of Freon are produced from how many moles of antimony trifluoride?
\(moles of antimony trifluoride=\frac{3*10^{6} moles of freon*2 moles of antimony trifluoride}{3 moles of freon}\)
moles of antimony trifluoride= 2*10⁶
The chemistry will need 2*10⁶ moles of antimony trifluoride.